1.
Introduction
Diabetic retinopathy is a complication of diabetes that affects a significant number of people worldwide. Diabetic retinopathy adversely impacts the retinal blood vessels, leading to eye complications. It may lead to vision problems and may cause blindness if left untreated. This condition affects approximately one-third of people with diabetes, and its prevalence increases with the duration of diabetes [1]. According to the American Diabetes Association, around 28% of people with diabetes over the age of 40 have diabetic retinopathy [2].
Blood vessel segmentation is an important step in analyzing retinal images because it separates blood vessels from the surrounding tissue. Manual labeling of blood vessels in medical images is a labor-intensive process that involves experts manually delineating vessel structures. Also, it is time-consuming as well as highly challenging for manual experts to detect small or complex blood vessels.
There are a lot of challenges faced during blood vessel segmentation namely: (a) Blood vessels often have low contrast compared to the background, making it difficult to distinguish them accurately; (b) since blood vessels often overlap with each other, it is difficult to separate individual vessels; and (c) the presence of vessels with different widths and branching patterns adds complexity to the segmentation task.
Addressing these challenges requires the development of robust segmentation algorithms that can handle variations in vessel appearance, structure, and imaging conditions. Thresholding [3,4], edge-based techniques [5,6], mathematical morphology [7,8], graph-based techniques [9], machine learning [10], and deep learning [11] are a few methods used to accurately segment blood vessels. Machine learning techniques such as SVM [12], Random Forest [13], and CNN [14] have shown promising results in segmentation. However, the U-Net architecture and its variants have recently shown better performance in blood vessel segmentation. Hence, effective attention with multi-scale learning residual U-Net (EAMR-Net) is proposed to enhance the effectiveness of retinal blood vessel segmentation. The major contributions are:
1). Residual block is employed instead of convolution block to avoid vanishing gradients and drop block is added after the convolution block to prevent overfitting.
2). A novel attention mechanism is introduced in the decoder block to treat all features uniquely. An effective spatial and channel attention mechanism is employed to preserve dimensionality and learn important features and interactions between channels in a cross-channel manner. Effective attention focuses on more information regions and ignores the background information.
3). A multi-scale feature learning (MSFL) module is proposed instead of the skip connection to avoid the semantic gap issue and enhance the model's ability to capture intricate details. Extracting hierarchical information will help to identify both large and finer details. To obtain image-level features, we add average pooling and up-sampling blocks. In comparison with the existing methods, the performance of our work has shown better accuracy.
The remaining sections of the paper are organized as follows: In Section 2, various research studies on retinal vascular segmentation are discussed. Section 3 provides an in-depth description of the research methodology and experimental design, while Section 4 discusses the experimental results obtained and ablation studies. Last, Section 5 provides a comprehensive summary and final remarks on the study.
2.
Related works
Based on the availability of label information, vessel segmentation in retinal images can be classified into unsupervised (unlabeled) and supervised learning techniques.
2.1. Unsupervised learning techniques
Unsupervised techniques do not use manual annotations for detecting blood vessels. Graph-based methods, clustering methods, and deep learning methods such as autoencoders and GAN (Generative Adversarial Networks) are a few unsupervised approaches used to segment the retinal vessels.
Padmapriya et al. [15] used basic pre-processing techniques, primary curvatures, ISODATA algorithm, and labeling to segment blood vessels with higher accuracy. Muzammil et al. [16] applied pre-processing techniques and used top-hat morphological operations to eliminate the noise. Matched and Gabor wavelet filters are applied to extract vessels. Then, binarization is applied using the human visual system. After applying a few post-processing procedures, the segmented results are produced. Qaiser et al. [17] suggested Otsu's thresholding method to create an automated retinal segmentation strategy that made use of pre-processing methods such as G-channel extraction, CLAHE, PCA, filtering, and segmentation. Upadhyay et al. [18] implemented two different varied scale transform approaches namely local directional wavelet and global curvelet. To recover boundary pixels, morphological thickness correction is proposed.
2.2. Supervised learning techniques
Initially, the U-Net architecture has shown a better performance. Few researchers have focused on building lightweight models and few others focused on performance improvement.
Ronneberger et al. [19] have developed a U-Net architecture that has shown better performance in biomedical segmentation. Laibacher et al. [20] proposed a new architecture that employs M2Unet as an encoder and contractive bottlenecks in the decoder part. Their focus is to reduce the parameter size used for training. This model is suitable for high-resolution image analysis. Boudegga et al. [21] proposed a model with convolution blocks of lightweight to reduce the computational complexity in terms of execution time. His model has shown better segmentation performance. Yang et al. [22] evaluated the performance of DCU-Net on three publicly available datasets and found that it had a lower number of parameters and a faster inference time.
Recently, an attention-based U-Net was developed to detect blood vessels accurately. Wang et al. [23] introduced a new deep learning architecture for retinal vessel segmentation called the Attention Inception-based U-Net- Advanced Residual (AIU-AR). The proposed model incorporates the attention mechanism and residual connections to enhance the accuracy of vessel segmentation in retinal images. The AIU-AR model has exhibited exceptional performance in comparison to several cutting-edge segmentation models when assessed using publicly accessible datasets. Through the incorporation of attention mechanisms and residual connections, researchers observed significant improvements in accuracy.
Wang et al. [24] developed a model, namely SAU-Net, that incorporates ResBlock along with inception blocks in the encoder part and, ResBlock with squeeze and excitation in the decoder part to eliminate the impact of hard exudate in fundus images. Dong et al. [25] proposed a model named CRAUnet which is a cascaded structure architecture that uses a residual block and an attention block. They also added a drop block to solve overfitting issues. Their contribution is multiscale feature extraction. Liu et al. [26] developed a ResDOU-Net that uses residual overparameterized convolution block, pooling fusion block, and atrous convolution to extract features in multiple scales, which demonstrated robustness to noise. Ren et al. [27] use Bi-FPN in U-Net, which uses depth-wise separable convolution for multiscale fusion. Li et al. [28] proposed GDFNet, which uses an enhancement network block to detect thin vessels, a global segmentation network to extract global features, and an attention fusion network that combines both. They also addressed information loss issues.
Liu et al. [29] introduced a WAVE-Net architecture that uses a denoising module to acquire microstructures and fuse contexts. The model addressed semantic loss and limited receptive field issues. Yi et al. [30] proposed MRANet that uses a feature fusion block to collect useful information, an attention block for better feature extraction, and a drop block for overfitting. Researchers provided the solution for segmenting capillary vessels and weak anti-noise interference ability.
Kumar et al. [31] proposed IterMiU-Net, which has iterative modules of Iternet. It is helpful to construct a lightweight convolution model with fewer parameters. The proposed IterMiUnet architecture is computationally efficient and can be used for real-time segmentation applications. Liu et al. [32] introduced DARes2Unet, which uses spatial attention and a dual attention model to address multiscale information and avoid unnecessary information.
Sun et al. [33] proposed SDAU-Net, which uses a series of deformable convolution and dual attention modules. It detects small vessels and solves the uneven brightness of the background. Li et al. [34] use MAGF-Net, which uses a residual block, an attention-guided fusion block and addresses information loss issues.
2.3. Motivation
U-Net model and its variations have shown better performance when compared with convolutional neural networks. However, most of the architectures fail to address the sensitivity rate problem, extracting multiscale feature extraction, information loss issues and segmenting thin vessels. Moreover, the computational complexity for most of the models is high.
Due to the above reasons, we are motivated to use drop block regularization in the residual block to prevent overfitting and added a multi-scale feature learning module that can extract features in multiple scales which is expected to capture intricate details. Also, adding effective attention in the decoder block can help to focus on more information regions and ignore the background information. This approach is expected to give a more powerful and context-aware learning model.
3.
Research methodology
Accurate segmentation of blood vessels is a highly challenging endeavor. The proposed architecture uses Residual U-Net as its baseline since it has demonstrated notable performance in various image segmentation processes [19]. The skip connection in U-Net reduces the information loss issue but introduces a semantic gap issue when the low-level features are combined with the high-level features. Therefore, an atrous spatial pyramid pooling block is proposed instead of the skip connection.
3.1. Architecture
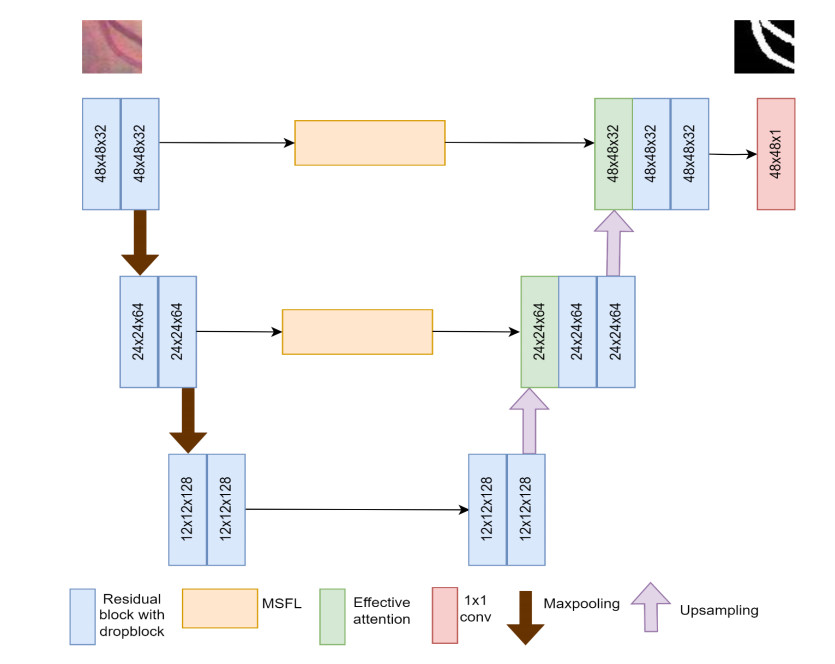
Each encoder block includes two residual blocks and max pooling operations. In the encoder phase, the extraction of features is carried out iteratively using pairs of consecutive residual blocks. Each pooling layer diminishes the spatial dimension. To expand the receptive field and capture broader contextual information, down sampling becomes essential. This downscaling operation is achieved by employing a pooling operation and a stride of 2 is used. During each down sampling process, the size of the feature maps is reduced by half, while the number of channels is doubled. Starting from initial feature maps of size 48 × 48, after two down sampling stages, they become 12 × 12 in dimensions. Further down sampling could lead to the loss of critical spatial information. Consequently, the network is designed with only two down sampling stages, featuring channel counts of 32, 64 and 128 in each stage.
The decoder block includes an up-sampling layer followed by a dual attention block and two residual blocks. For each up-sampling layer, bilinear interpolation is used to maintain the size of the feature map. The convolutional layers are configured with channel numbers 32, 64, and 128. The architecture of EAMR-Net is depicted in Figure 1.
3.1.1. Residual block
The residual block [35] has shortcut connections that may skip a few layers and will perform identity mapping without increasing computational complexity. It facilitates faster convergence, reduces the impact of vanishing gradients, and reduces the risk of losing important information by encouraging feature reuse. A residual block consists of multiple layers that are arranged sequentially, allowing them to pass their output to a subsequent layer positioned deeper within the block. Residual blocks address the issue of gradient vanishing by introducing skip-connections. The residual block contains two sequences of convolution, drop block, batch normalization (BN), and ReLU. The convolution operation is carried out on a skip connection. Finally, the residual block is added with a skip connection, which does identity mapping as shown in Figure 2. Drop block is a technique reminiscent of dropout, differing in that it excludes entire contiguous regions from a layer's feature map, as opposed to independently removing random individual units. Drop block retains the spatial information and helps to alleviate issues like overfitting and vanishing gradients. In the residual module, it is proven that placing the drop block [36] after the convolution block improves accuracy.
Let xr represent the input of the residual block, S(xr) represent the skip connection path output and R(xr) represent the residual path output, Then the output of residual block yr is obtained as
The residual path output R (xr) is represented as
The skip connection path output S(xr) is represented as
3.1.2. MSFL module
The MSFL module [37,38] employs multiple atrous convolutions to find deep features parallelly. To extract multiscale features [39] and large receptive fields [40], these convolution layers have different sampling (dilation) rates. This is a better approach for controlling the field of view without increasing parameters and computational time.
The input feature map is passed to the 1 × 1 convolution layer, followed by three atrous 3 × 3 convolution layers with different dilation rates (6, 12 and 18). In addition, we add average pooling and upsampling blocks to obtain image-level features. Finally, the output of these parallel branches is combined using the concatenation operator. Then, 1x1 convolution is applied to the final feature map by fusing the feature maps of MSFL as depicted in Figure 3. The major shortcoming of MSFL is that it will treat all feature scales equally [41]. To tackle this issue, an attention block is introduced after the MSFL blocks.
3.1.3. Effective attention bblock
The effective attention block (Figure 4) incorporates both spatial and effective channel attention. The spatial attention block assigns higher weights to important features and diminishes the weights of redundant features, thereby enabling effective learning [42]. To the input feature map (F), both max pooling and average pooling operations are performed, followed by a 7 × 7 convolution and a sigmoid operation. This attention map obtained gives more precise spatial information.
where σ represents a sigmoid activation function.
The spatial attention map is multiplied by the input feature to produce the output feature.
where ⨂ represents element-wise multiplication.
Effective channel attention involves dynamically adjusting the characteristics of individual channels in a feature representation to adaptively recalibrate their relevance. Squeeze and excitation (SE) [43] block use a dimensionality reduction mechanism to obtain channel-wise descriptors that diminish the complexity of the model but break the direct relationship between a channel and its corresponding weight. To mitigate this issue, ECANet [44] is employed for channel attention, which preserves the dimensionality to learn important features and does interactions between channels in a cross-channel manner.
Therefore, we apply Global Average Pooling (GAP) to capture global information by taking average values across spatial dimensions. To obtain the channel descriptor space, forward convolution is applied with ReLU activation. Then, channel attention is done by fully sigmoid activation, which produces a channel attention weight that describes the significance of each channel relative to the other. Finally, recalibration is done to rescale each channel's activation adaptively.
where k represents the size of the kernel of 1D convolution which is determined adaptively and is proportional to the dimension of the channel.
The new channel feature maps are procured by reassigning weights:
where ⊗ represents channel-wise multiplication.
The final output feature of effective attention is obtained by multiplying the channel feature with the spatial feature.
The EAMR-Net architecture summary is tabulated in Table 1. The table displays the name of the layer and image size of each block used in the architecture.
3.2. Experimental setup
3.2.1. Datasets used
1). DRIVE: The DRIVE (https://drive.grand-challenge.org/) dataset consists of 40 images collected from 400 patients with a manual annotation equally split for training and testing. Out of the total dataset, consisting of 40 images, 33 are categorized as normal cases, while the remaining 7 exhibit indications of diabetic retinopathy. Each image is of 565 × 584 resolution. The images are captured with a 3CCD camera with a 45° field of view (FOV). The images are in TIFF format and the manual annotations are stored as GIF files.
2). CHASE_DB1: CHASE_DB1 (https://blogs.kingston.ac.uk/retinal/chasedb1/) has 28 images captured from 14 children with 999 × 960 resolution. The images are captured with a Nidek NM-200-D fundus camera, 30-degree FOV. Since images need to be categorized as train and test, the initial set of 20 images will be utilized for training, while the remaining images will be reserved for testing. Two manual annotations are given. We have considered the first manual labelling as ground truth. The images are in JPEG format and manually labelled images are in PNG format.
3). STARE: The STARE (https://cecas.clemson.edu/~ahoover/stare/) dataset contains 20 color fundus images and a manually labeled segmentation mask. Out of a total of 20 images, 10 exhibit retinal abnormalities associated with diabetic retinopathy, while the remaining 10 images show no indications of this condition. Each image has a resolution of 700 × 605. The images are captured with Top con TRV-50 fundus camera 35-degree FOV. Both the image and labelled image are in JPEG format. In the training phase, the initial set of 15 images is utilized, while during the testing phase, the remaining set of 5 images is employed for evaluation.
3.2.2. Preprocessing
Preprocessing is a fundamental aspect of image processing that significantly improves image quality, reduces noise, enhances contrast, standardizes image properties, and extracts pertinent features. These preprocessing steps are critical for ensuring precise and dependable analysis, interpretation, and decision-making across diverse fields like computer vision, medical imaging, remote sensing, and other relevant domains. During the preprocessing phase, techniques such as normalization, contrast adjustment (CLAHE), and gamma transformation are carried out. Figure 5 depicts the result of a sample fundus image after each preprocessing stage.
To alleviate the problem of overfitting due to the limited dataset, the images are divided into 48 × 48 patches using a random cropping technique. Image resolution and size of the input patch influence the level of detail the model can capture. Smaller patch sizes can capture fine details. Moreover, the computational resources and memory constraints have a greater influence in determining the image size. Then, we applied data augmentation techniques such as horizontal flipping, vertical flipping, elastic transform, grid distortion, rotation at an angle of 30°, 60°, random brightness, and random contrast.
3.2.3. Evaluation metrics
For quantitative analysis of segmentation results, several metrics used are Accuracy (ACC), Specificity (SPEC), Sensitivity (SEN), F1 score (F1), and Area under Receiver Operating Characteristic (AUC) curve.
ACC: It tells us how exactly the vessels and non-vessels are predicted.
SEN: It specifies the proportion of correctly predicted blood vessels to actual blood vessels.
SPEC: It represents the ratio between the accurately detected non-blood vessels and the total number of non-blood vessels.
F1 score (F1): It considers both the ability to correctly identify blood vessels (precision) and its ability to capture all actual blood vessels (recall).
AUC: It tells how well the blood vessels and non-blood vessel segmentation is done.
TN, TP, FN, and FP represent true negative, true positive, false negative, and false positive, respectively.
3.3. Implementation details
The algorithm utilizes the Keras library from the TensorFlow backend and is executed on an NVIDIA RTX3060, equipped with 12 GB of memory. Pre-trained model is not used in our experimentation. The initialization of the algorithm is performed using Glorot uniform weights.
The algorithm employs the Adam optimizer with an initial learning rate of 0.001. It utilizes a binary cross-entropy loss function for training. The number of epochs used during the training process is 100. Due to limited memory, a batch size of 8 is used.
3.3.1. Loss function
The binary cross-entropy loss function quantifies how well a model's predicted probabilities align with the actual binary labels (0 or 1) for each example, particularly focusing on cases where the true label is 1.
Mathematically, the binary cross-entropy loss function is defined as:
where, Loss BCE is the loss function, N is the total number of pixels, gti is the true binary label (ground truth), and pi is the predicted probability that the example belongs to class 1.
3.3.2. Network hyperparameter analysis
The hyperparameters such as architecture depth, loss function, learning rate, and optimizer play a crucial role in improving the accuracy of segmentation. In the research studies, Adam optimizer and BCE loss function are employed. If the model is down-sampled further from 12 × 12, there is a loss of more spatial information. So, the layer depth is been fixed to three.
The segmentation outcomes are significantly influenced by the choice of learning rate. Adjusting the learning rate has a pronounced impact on the final segmentation results. Table 2 lists the evaluation models with different learning rate on the dataset DRIVE.
When the learning rate is 0.001, our proposed model obtained higher accuracy and sensitivity. With a learning rate set at 0.003, the model exhibits poor performance. At learning rates of both 0.0001 and 0.00001, the model has demonstrated relatively modest performance.
As shown in Figure 6, during the training stage, there is a gradual improvement in training and validation accuracy as the epoch increases.
4.
Experimental results
4.1. Ablation studies
To track the model's effects, various ablation studies are conducted on three datasets by adding new blocks to an existing architecture. We used Residual U-Net as the baseline and compared the segmentation results with EA+ResU-Net and EAMR-Net. The proposed model has shown better performance when compared with the other models in ablation studies, as shown in Figure 7.
We set the drop rate value as 0.1 and the block size as 7. When residual U-Net with a drop block is employed, we observed some discontinuous segmentation in overlapping areas of vessels. Then, an efficient dual attention block is added to the residual U-Net which shows improvement in the detection of vessels in overlapping areas. However, due to the low contrast in the background, it was unable to distinguish a few blood vessels. So, instead of using a skip connection, an MSFL block is added, and we found that the performance is superior to other models in detecting vessels in low contrast as well as overlapping areas.
The ablation study results for different datasets are tabulated in Table 3. In the DRIVE dataset, our model has shown a 1.98% increase in sensitivity, which reports that there is an improvement in blood vessel detection. Specificity, accuracy, and the F1 score have also shown improvement. However, in DRIVE and CHASE_DB1, there is a slight decrease in specificity when an effective attention block is added. This behavior is due to poor contrast in a few images in the dataset. In the STARE dataset, sensitivity has improved by 1.45%. Also, there is an improvement in results in ACC, SPEC, and AUC curves. The CHASE_DB1 dataset has also shown progress in detecting blood vessels. There is a 1.26% increase in sensitivity for the proposed model.
4.2. Comparative analysis with other models
We compared our model with some recent models like R2U-Net [45], DCU-Net [22], CRAU-Net [25], ResDOU-Net [26], GDF-Net [28], Wave-Net [29], MAGF-Net [34], IterMiU-Net [31] and SDAU-Net [33]. The EAMR-Net model has shown better performance in specificity, sensitivity, and the AUC compared with state-of-the-art models.
The findings of the experiment on DRIVE, STARE, and CHASE_DB1 are tabulated in Table 4. Our model has shown better performance on SPEC and AUC. Its sensitivity is the same as the SDAU-Net model. Our model reaches nearly 0.9853 on SPEC and 0.9861 on AUC. The SPEC is 0.0050 higher than R2U-Net and AUC is 0.0002 higher than ResDOU-Net.
On the STARE dataset, the proposed model has achieved 0.9713 ACC, 0.9932 SPEC, 0.8151 SEN, 0.9881 AUC, and 0.8254 F1 scores. It has achieved a 0.0004 increase in specificity when compared to RV-Net.
In CHASE_DB1, experimental results show that the ACC, SPEC, SEN, AUC, and F1 score are 0.9665, 0.9848, 0.8084, 0.9875 and 0.8423 respectively. In comparison with other methods, it has achieved a specificity of 0.0007 higher than DCU-Net.
The qualitative analysis is done on the three benchmark datasets. Figure 8 presents a visual comparison of our model, EAMR-Net with other state-of-art models. We have chosen ResU-Net, AttResU-Net, and RCARU-Net for qualitative analysis. Upon examining the detailed regions, it becomes evident that ResU-Net, AttResU-Net, and RCARU-Net [46] possess the capability to extract the retinal vessels from the original image. However, a closer inspection of the ground truth images reveals noticeable disconnections and mis-segmentations in the capillary region. In this comparison, EAMR-Net stands out for its proficiency in detecting capillary vessels, and the demonstration of excellent connectivity between vessels. It is observed that the area shown in the red rectangular box shows the segmentation of thin vessels. Compared to other techniques, the EAMR-Net method has performed better.
5.
Conclusions
A novel architecture with effective attention and multiscale learning is proposed to detect blood vessels precisely. To prevent overfitting, a drop block is appended after every convolution block, and residual U-net is used to prevent vanishing gradients. To preserve the dimensionality of spatial and channel features, a dual attention block is used. To avoid the semantic gap issue, an MSFL block is added. The effectiveness of the suggested approach is compared with the existing method in three datasets namely, DRIVE, STARE and CHASE_DB1, and has achieved better results. The sensitivity of 0.8293, 0.8151, and 0.8084 is attained on the DRIVE, STARE and CHASE_DB1 datasets respectively. In future research and development, one exciting avenue to explore is the integration of vision transformers (ViTs) into model design. Also, Generative Adversarial Networks (GANs) can be employed for enhancing image augmentation techniques.
Use of AI tools declaration
The authors declare that they have not used Artificial Intelligence (AI) tools in the creation of this article.
Conflict of interest
The authors declare that there are no conflicts of interest.









 DownLoad:
DownLoad: