1. Introduction
In human history, over 70 % of the emerging infectious diseases are zoonoses, which mainly originate from animal reservoirs. Zoonotic pathogens can transmit from animals to humans. And about 75 % of these zoonotic pathogens originate from wildlife [28,3,24]. Wildlife, domestic animals and humans construct the network of pathogen transmission crossing the species barrier. Wildlife and domestic animals play important roles in the transmission of zoonotic pathogens, in spite of the fact that we always neglected them before a zoonosis emerging or reemerging [12,4].
No matter how well the science and technology developed in human society, human is just one kind of animals, even though other animals are not equal to humans in living status. The existence of the humans has changed the relationship between humans and animals due to some anthropogenic factors. Humans domesticated wolf, which was the ancestor of dog, for hunting about tens of thousands of years ago. Later, the intimacy between humans and dogs was increased more and more by natural selection or human selection, to be precise. In the meantime rabies virus existed permanently in human life by dog-human interface maintaining, as dogs were the mainly natural reservoirs of them, especially in Asia[24,32].
Animals are divided into wildlife and domestic animals by human selection [24]. Humans can manage domestic animals in their entire life, but they cannot control wildlife at liberty. At the same time, humans can contact with domestic animals sufficiently, but they have few opportunities to get in touch with wildlife except for some special professions, such as forest conservationists and poachers. As wildlife and domestic animals play different roles in human life, the zoonotic pathogen transmissions in wildlife infection, domestic animal infection and human infection would be in different styles [17,27]. Various mathematical models have been established in the study of zoonoses [26,1,16,31,11,29]. For example, Doctor Saenz and his partners discussed the impact of domestic animal-human interface in pathogen transmission [26] and Doctor Allen constructed several types of mathematical models to reflect the pathogen transmission in wildlife [1].
For pathogen transmission in multiple species, the multi-SIR model can be established as the form [1,17]:
|
{˙Si=Ai−n∑j=1βjiIjSi−μiSi,˙Ii=n∑j=1βjiIjSi−μiIi−γiIi−αiIi,˙Ri=γiIi−μiRi.
|
(1)
|
Si, Ii and Ri represent the number of susceptibles, infectives, and recovered individuals for species i, i=1, 2, ⋯, n. Ai is the birth or immigration rate for species i. μi is the natural mortality rate. αi is the disease-induced mortality rate. γi is the recovery rate. And βji is the per capita incidence rate from species j to species i, which denotes the probability of Ij infecting Si.
The basic reproduction number R0 for model (1) is the spectral radius of [R0(ji)]n×n, where R0(ji)=Aiβjiμi(μi+γi+αi) [10]. For βji≠0, ∀ j,i, it is difficult to get R0 clearly. So we can take some biological characteristics of wildlife, domestic animals and humans into account to limit the value of βji in order to simplify [R0(ji)]n×n.
For wildlife, they are always the origin of animal-borne zoonoses [24,12]. The pathogen transmission from wildlife to humans is often neglected due to geographic distance between them, but the globalization and urbanization has shortened this distance. The linkage between wildlife and humans is established with anthropogenic land expanding[24]. And pathogens parasitized in different species could be transmitted to others crossing species barrier by this linkage. But for emerging zoonoses, wildlife play as the only role of natural reservoirs. The pathogen transmission from domestic animals to wildlife or from humans to wildlife could not cause emerging zoonoses. Because the pathogens parasitized in humans or domestic animals have already existed for a period of time, which could be not defined as an emerging event even if the pathogens might transmit back to humans. For example, Severe Acute Respiratory Syndromes (SARS) is defined as an emerging zoonosis, which originate from Rhinolophus, then transmit via palm civets as intermediate host to humans [8]. But for mycobacterium tuberculosis, taking humans as their reservoirs, it could not give rise to an emerging zoonosis even if it had opportunities to infect other animals [20].
That is to say, for wildlife, the zoonotic pathogens could transmit in them, βWW≠0, but βHW=0 and βDW=0. Here we classify the hosts into three groups: wildlife, domestic animals and humans. And notation W presents wildlife, D presents domestic animals and H presents humans. In order to simplify the model further, we assume that the zoonotic pathogen transmission could not occur from humans to domestic animals in emerging event. The need of infected people is to have a rest, but not to take care of other animals [24,26]. We assume that if an emerging zoonosis was prevalent in human life, people could be infected from other people without the need of passing by domestic animals, then to humans. So βHD=0, but βDD≠0 and βWD≠0.
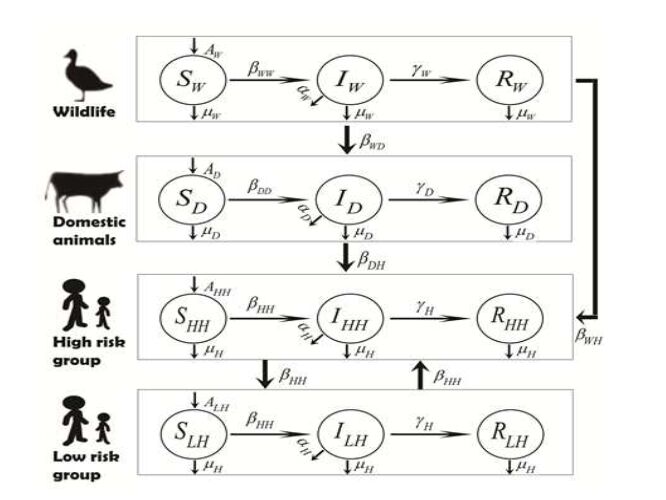
For the relationship between animals and humans, we assume that not all of people could have opportunities to be infected from animals. Live animals are the mainly origin of zoonotic pathogens and only part of people could contact with them including CAFO (Confined Animal Feeding Operation) workers and hunters [26]. We also take the human population heterogeneity into consideration in this paper. The human population is classified into two groups: high risk group and low risk group. High risk group has the opportunities to contact with infected animals sufficiently. But low risk group are the others. That is, high risk group can get pathogens from animals and humans, but low risk group from humans only. The emerging zoonotic pathogen transmission can be described in FIGURE 1.
Emerging zoonotic pathogen transmission from wildlife, to domestic animals, to humans can be described as the model (2).
Si, Ii and Ri represent the number of susceptibles, infectives, and recovered individuals for wildlife with i=W, domestic animals with i=D, high risk group with i=HH and low risk group with i=LH. Ai is the birth or immigration rate for species i=W, D, HH or LH. μi, γi, and βji are defined as the same as model (1) with i=W,D, or H. Here we assume that recovered individuals could be immune in a period time when a novel zoonosis is emerging.
|
{˙SW = AW−βWWIWSW−μWSW,˙IW=βWWIWSW−(μW+γW+αW)IW,˙RW=γWIW−μWRW,˙SD=AD−(βWDIW+βDDID)SD−μDSD,˙ID=(βWDIW+βDDID)SD−(γD+αD+μD)ID,˙RD=γDID−μDRD,˙SHH = AHH−[βWHIW+βDHID+βHH(IHH+ILH)]SHH−μHSHH,˙IHH=[βWHIW+βDHID+βHH(IHH+ILH)]SHH −(γH+αH+μH)IHH,˙RHH=γHIHH−μHRHH,˙SLH = ALH−βHH(IHH+ILH)SLH−μHSLH,˙ILH=βHH(IHH+ILH)SLH−(γH+αH+μH)ILH,˙RLH=γHILH−μHRLH.
|
(2)
|
The basic model has been established to reflect the pathogen transmission from wildlife, to domestic animals, to humans as model (2). Next step, we take the isolation and slaughter strategies into consideration [22,23,8,31,2,25,18]. For wildlife, it is difficult to control them when a zoonosis is emerging. Lethal control, vaccination and fencing (physical barriers) are the primary approaches to limit the number of susceptibles in wildlife. In this paper, we take lethal control and fencing (physical barriers) as the strategies to compare the similar isolation and slaughter strategies in emerging zoonotic pathogen transmission.
|
{˙SW = AW−βWWIWSW−(μW+δS)SW,˙IW=βWWIWSW−(μW+γW+αW+δI)IW,˙RW=γWIW−(μW+δR)RW,˙SD=AD−((1−θD)βWDIW+βDDID)SD−μDSD,˙ID=((1−θD)βWDIW+βDDID)SD−(γD+αD+μD)ID,˙RD=γDID−μDRD,˙SHH = AHH−[(1−θH)βWHIW+βDHID+βHH(IHH+ILH)]SHH −μHSHH,˙IHH=[(1−θH)βWHIW+βDHID+βHH(IHH+ILH)]SHH −(γH+αH+μH)IHH,˙RHH=γHIHH−μHRHH,˙SLH = ALH−βHH(IHH+ILH)SLH−μHSLH,˙ILH=βHH(IHH+ILH)SLH−(γH+αH+μH)ILH,˙RLH=γHILH−μHRLH.
|
(3)
|
δs, δI and δR represent lethal control or slaughter rate of susceptibles, infectives, and recovered individuals in wildlife. θD, θH represent effectiveness of fencing (physical barriers), θD,θH∈[0,1]. If θD=1, θH=1, fencing plays the best role in the control of emerging zoonoses. If θD=0, θH=0, fencing is useless in the control of emerging zoonoses.
For domestic animals, we can manage them in their entire lives. It is no need to slaughter all of the susceptibles in domestic animals. We can quarantine all of the domestic animals, then isolate susceptibles and slaughter infectives.
|
{˙SD=AD−(βWDIW+βDDID)SD−μDSD,˙ID=(βWDIW+βDDID)SD−(γD+αD+μD+ΔI)ID,˙RD=γDID−μDRD,˙SHH = AHH−[βWHIW+(1−ΘH)βDHID+βHH(IHH+ILH)]SHH −μHSHH,˙IHH=[βWHIW+(1−ΘH)βDHID+βHH(IHH+ILH)]SHH −(γH+αH+μH)IHH,˙RHH=γHIHH−μHRHH,˙SLH = ALH−βHH(IHH+ILH)SLH−μHSLH,˙ILH=βHH(IHH+ILH)SLH−(γH+αH+μH)ILH,˙RLH=γHILH−μHRLH.
|
(4)
|
ΔI represents slaughter rate of infectives in domestic animals. ΘH represents effectiveness of isolation, ΘH∈[0,1]. If ΘH=1, isolation from susceptible domestic animals play the best role in the control of emerging zoonoses. If ΘH=0, isolation in domestic animals is useless in the control of emerging zoonoses.
For humans, we could not 'slaughter' anyone no matter how serious they were infected with some kind of zoonoses. The quarantine and isolation may be the best method to limit the pathogen transmission except for vaccination. But the effect of quarantine and isolation strategies in humans are different from animals. For taking isolation strategies in animals, it is the susceptible humans, who are afraid of getting infected, to take the initiative and get away from susceptible animals. So the per capita incidence rate from animals to humans, βWH and βDH, is decreased by θH and ΘH. But in humans, we quarantine and isolate the infected people to cut off pathogen transmission way. βHH would not change at this time, but there is a new compartment O produced, which denotes the isolation compartment [25].
|
{˙SHH = AHH−[βWHIW+βDHID+βHH(IHH+ILH)]SHH −μHSHH−φ(I)SHH+γH1OHH1,˙OHH1=φ(I)SHH−γH1OHH1−μHOHH1,˙IHH=[βWHIW+βDHID+βHH(IHH+ILH)]SHH −(γH+αH+μH+σ)IHH,˙OHH2=σIHH−γH2OHH2−μHOHH2,˙RHH=γHIHH+γH2OHH2−μHRHH,˙SLH = ALH−βHH(IHH+ILH)SLH−μHSLH −φ(I)SLH+γH1OLH1,˙OLH1=φ(I)SLH−γH1OLH1−μHOLH1,˙ILH=βHH(IHH+ILH)SLH−(γH+αH+μH+σ)ILH,˙OLH2=σILH−γH2OLH2−μHOLH2,˙RLH=γHILH+γH2OLH2−μHRLH.
|
(5)
|
OHH1 and OHH2 represent isolation compartments from susceptibles and infectives in high risk group respectively. OLH1 and OLH2 represent isolation compartments from susceptibles and infectives in low risk group. Susceptibles enter the OHH1 and OLH1 classes at the rate of φ(I)SHH and φ(I)SLH, with φ(I)=ρ(IHH+ILH) [23]. The infectives are isolated at the constant per-capita rate of σ. γH1 is the remove rate from isolation compartment to susceptible compartment.γH2 is the remove rate from isolation compartment to recovery individual compartment.
In conclusion, we can get the isolation and slaughter strategies controlling model by (3), (4) and (5) in wildlife, domestic animals and humans as the form:
|
{˙SW = AW−βWWIWSW−(μW+εWδS)SW,˙IW=βWWIWSW−(μW+γW+αW+εWδI)IW,˙RW=γWIW−(μW+εWδR)RW,˙SD=AD−((1−εWθD)βWDIW+βDDID)SD−μDSD,˙ID=((1−εWθD)βWDIW+βDDID)SD −(γD+αD+μD+εDΔI)ID,˙RD=γDID−μDRD,˙SHH = AHH−[(1−εWθH)βWHIW+(1−εDΘH)βDHID+βHH(IHH +ILH)]SHH−μHSHH−εHφ(I)SHH+γH1OHH1,˙OHH1=εHφ(I)SHH−γH1OHH1−μHOHH1,˙IHH=[(1−εWθH)βWHIW+(1−εDΘH)βDHID +βHH(IHH+ILH)]SHH−(γH+αH+μH+εHσ)IHH,˙OHH2=εHσIHH−γH2OHH2−μHOHH2,˙RHH=γHIHH+γH2OHH2−μHRHH,˙SLH = ALH−βHH(IHH+ILH)SLH−μHSLH −εHφ(I)SLH+γH1OLH1,˙OLH1=εHφ(I)SLH−γH1OLH1−μHOLH1,˙ILH=βHH(IHH+ILH)SLH−(γH+αH+μH+εHσ)ILH,˙OLH2=εHσILH−γH2OLH2−μHOLH2,˙RLH=γHILH+γH2OLH2−μHRLH.
|
(6)
|
with Strategy 1,
|
{εW=0,ILH+IHH<IWCεW=1,ILH+IHH≥IWC
|
(7)
|
Strategy 2,
|
{εD=0,ILH+IHH<IDCεD=1,ILH+IHH≥IDC
|
(8)
|
Strategy 3,
|
{εH=0,ILH+IHH<IHCεH=1,ILH+IHH≥IHC
|
(9)
|
The feasible set Ω={(SW(t), IW(t), RW(t), SD(t), ID(t), RD(t),SHH(t), OHH1(t), IHH(t), OHH2(t), RHH(t),SLH(t), OLH1(t), ILH(t), OLH2(t),RLH(t))∣Si(t),Ii(t),Ri(t),Oj(t)≥0, 0<N≤AWμW+ADμD+AHHμH+ALHμH,i=W,D,HH,LH,j=HH1,HH2,LH1,LH2} is the positively invariant with respect to (6).
Total number of wildlife is NW=SW+IW+RW. Total number of domestic animals is ND=SD+ID+RD. Total number of humans is NH=SHH+OHH1+IHH+OHH2+RHH+SLH+OLH1+ILH+OLH2+RLH. Total number of susceptible humans is SH=SHH+SLH. Total number of infective humans is IH=IHH+ILH. Total number of recovery humans is RH=RHH+RLH. Total number of all is N=NW+ND+NH.
It is difficult for us to take any strategies to control emerging zoonoses in first time. Only the infected of numbers of people would cause our attention to take some strategies to control the infectious disease. So it is assumed that if the number of infectives in human including high risk group and low risk group reached a threshold at IWC,IDC or IHC, we would take measures as Strategy 1 in wildlife, Strategy 2 in domestic animals or Strategy 3 in humans.
2. Stability analysis
With εW=0,εD=0,εH=0, we can get (2) before taking any measures in emerging zoonoses. At first, we analyze the equilibrium stability of model (2) [5,14,19,16].
In (2), the wildlife class can be separated as
|
{˙SW = AW−βWWIWSW−μWSW,˙IW=βWWIWSW−μWIW−γWIW−αWIW.
|
(10)
|
We can get the basic reproductive number in wildlife R0(WW)=AWβWWμW(μW+γW+αW).
The disease-free equilibrium is E0(W)=(¯SW,¯IW)=(AWμW,0), the epidemic equilibrium is E∗(w)=(ˆSW,ˆIW)=(μW+γW+αWβWW,μWβWW(AWβWWμW(μW+γW+αW)−1)). The number of recovery individuals would not change the stability of the system, so we neglect it in the study of equilibriums.
Theorem 2.1. If R0(WW)<1, the disease-free equilibrium E0(W) in wildlife is stable. If R0(WW)>1, the epidemic equilibrium E∗(W) in wildlife is stable.
Proof. The next generation matrix of the vector field corresponding to system (10) at E0(W) is
|
JW(E0(W))=(−μW−βWWAWμW0βWWAWμW−μW−γW−αW)
|
If R0(WW)=AWβWWμW(μW+γW+αW)<1, the eigenvalues of JW(E0(W)) are negative and E0(W) is stable.
Similarly, the next generation matrix at E∗(W) is
|
JW(E∗(W))=(−βWWˆIW−μW−βWWˆSWβWWˆIWβWWˆSW−μW−γW−αW)
|
The characteristic equation of JW(E∗(W)) is
|
fW(λ)=λ2+AWβWWμW+γW+αWλ+μW(μW+γW+αW)(AWβWWμW(μW+γW+αW)−1)=0
|
If R0(WW)=AWβWWμW(μW+γW+αW)>1, the real parts of eigenvalues of JW(E∗(W)) are negative and E∗(W) is stable.
In (2), the wildlife and domestic animals classes can be separated as
|
{˙SW = AW−βWWIWSW−μWSW,˙IW=βWWIWSW−μWIW−γWIW−αWIW,˙SD = AD−βWDIWSD−βDDIDSD−μDSD,˙ID=βWDIWSD+βDDIDSD−γDID−αDID−μDID.
|
(11)
|
We can get the basic reproductive number in domestic animals is R0(DD)=ADβDDμD(μD+γD+αD).
The disease-free equilibrium is E0(WD)=(¯SW,¯IW,¯SD,¯ID)=(AWμW,0,ADμD,0), the epidemic equilibrium is
E∗(WD)=(ˆSW,ˆIW,ˆSD,ˆID)=(μW+γW+αWβWW,μWβWW(AWβWWμW(μW+γW+αW)−1),ˆSD,ˆID).
Theorem 2.2. If R0(WW)<1 and R0(DD)<1, the disease-free equilibrium E0(WD) in system (11) is stable. If R0(WW)>1, there exists one unique positive epidemic equilibrium E∗(WD), and E∗(WD) is stable.
Proof. There always exists E0(WD) and the next generation matrix at E0(WD) is
|
JWD(E0(WD))=(JW(E0(W))0∗JD(E0(D)))
|
with
|
JD(E0(D))=(−μD−βDDADμD0βDDADμD−μD−γD−αD)
|
If R0(WW)=AWβWWμW(μW+γW+αW)<1 and R0(DD)=ADβDDμD(μD+γD+αD)<1, the eigenvalues of JWD(E0(WD)) are negative and E0(WD) is stable.
In (11), the epidemic equilibrium E∗(WD)=(ˆSW,ˆIW,ˆSD,ˆID) satisfies
|
AW−βWWˆIWˆSW−μWˆSW = 0
|
(12)
|
|
βWWˆIWˆSW−μWˆIW−γWˆIW−αWˆIW = 0
|
(13)
|
|
AD−βWDˆIWˆSD−βDDˆIDˆSD−μDˆSD = 0
|
(14)
|
|
βWDˆIWˆSD+βDDˆIDˆSD−μDˆID−γDˆID−αDˆID = 0
|
(15)
|
From (12), (13), (14), (15), we can get
|
ˆIW=μWβWW(AWβWWμW(μW+γW+αW)−1)
|
|
ˆID = βWDˆIWˆSDμD+γD+αD−βDDˆSD=βWDˆSDμD+γD+αD−βDDˆSD×μWβWW(AWβWWμW(μW+γW+αW)−1)
|
and
|
ˆSD=12μDβDD[ADβDD+(μD+γD+αD)(μD+βWDˆIW)]−12μDβDD[A2Dβ2DD+2ADβDD(μD+γD+αD)(βWDˆIW−μD)+(μD+γD+αD)2(μD+βWDˆIW)2]12
|
So if R0(WW)>1, there exists one unique positive epidemic equilibrium E∗(WD).
In fact, for ˆSD, ˆSD satisfies
|
g1(ˆSD)=μDβDDˆSD2−[ADβDD+(μD+γD+αD)(μD+βWDˆIW)]ˆSD+AD(μD+γD+αD)=0
|
If g1(ˆSD)=0, ˆSD always has two positive roots because of −ADβDD+(μD+γD+αD)(μD+βWDˆIW)<0, AD(μD+γD+αD)>0, and the smaller ˆSD and the bigger ˆSD stand on both sides of μD+γD+αDβDD for g1(μD+γD+αDβDD)<0 and μDβDD>0.
So we choose
|
ˆSD=12μDβDD[ADβDD+(μD+γD+αD)(μD+βWDˆIW)]−12μDβDD[A2Dβ2DD+2ADβDD(μD+γD+αD)(βWDˆIW−μD)+(μD+γD+αD)2(μD+βWDˆIW)2]12
|
to guarantee ˆID>0.
The next generation matrix at E∗(WD) is
|
JWD(E∗(WD))=(JW(E∗(W))0∗JD(E∗(D)))
|
with
|
JD(E∗(D))=(−βWDˆIW−βDDˆID−μD−βDDˆSDβWDˆIW+βDDˆIDβDDˆSD−μD−γD−αD)
|
The characteristic equation of JD(E∗(D)) is fD(λ)=λ2 + (ADˆSD + βWDˆIWˆSDˆID)λ+βWDˆIWADˆID + βWDβDDˆSDˆIW + βDD2ˆSDˆID=0.
If E∗(D) exists, all of the real parts of eigenvalues of JD(E∗(D)) are negative for ADˆSD + βWDˆIWˆSDˆID>0 and βWDˆIWADˆID + βWDβDDˆSDˆIW + βDD2ˆSDˆID>0.
In conclusion, if R0(WW)=AWβWWμW(μW+γW+αW)>1, the real parts of eigenvalues of JD(E∗(D)) are negative and E∗(WD) is stable.
The human class in (2) can be separated as the form:
|
{˙SHH = AHH−βWHIWSHH−βDHIDSHH−βHH(IHH+ILH)SHH −μHSHH,˙IHH=βWHIWSHH+βDHIDSHH+βHH(IHH+ILH)SHH−γHIHH −αHIHH−μHIHH,˙SLH = ALH−βHH(IHH+ILH)SLH−μHSLH,˙ILH=βHH(IHH+ILH)SLH−γHILH−αHILH−μHILH.
|
(16)
|
There always exists disease-free equilibrium E0(WDH)=(¯SW,¯IW,¯SD,¯ID,¯SHH,¯IHH,¯SLH,¯ILH)=(AWμW,0,ADμD,0,AHHμHH,0,ALHμLH) in (2).
The next generation matrix at E0(WDH) is
|
JWDH(E0(WDH))=(JW(E0(W))00∗JD(E0(D))0∗∗JH(E0(H)))
|
with
|
JH(E0(H))=(−μH−βHHAHHμH0−βHHAHHμH0βHHAHHμH−μH−γH−αH0βHHAHHμH0−βHHALHμH−μH−βHHALHμH0βHHALHμH0βHHALHμH−μH−γH−αH)
|
The characteristic equation of JH(E0(H) is
fH1(λ)=(λ+μH)2(λ2−(βHHAHH+ALHμH−2μH−2γH−2αH)λ−(μH+γH+αH)(βHHAHH+ALHμH−μH−γH−αH))=0
If there is R0(H)=(AHH+ALH)βHHμH(μH+γH+αH)<1, we have βHHAHH+ALHμH−2μH−2γH−2αH<0 and (μH+γH+αH)(βHHAHH+ALHμH−μH−γH−αH)<0. Then we get all of the real parts of eigenvalues of JH(E0(H)) are negative, E0(H) is stable.
At the same time, the spectral radius of [R0(HHHH)R0(LHHH)R0(HHLH)R0(LHLH)] is R0(H)=(AHH+ALH)βHHμH(μH+γH+αH), with R0(HHHH)=R0(LHHH)=AHHβHHμH(μH+γH+αH) and R0(LHLH)=R0(HHLH)=ALHβHHμH(μH+γH+αH).
Theorem 2.3. If R0(WW)<1, R0(DD)<1 and R0(H)<1, the disease-free equilibrium E0(WDH) in system (2) is stable. If R0(WW)>1, there exists epidemic equilibrium E∗(WDH), and E∗(WDH) is stable.
Proof. The next generation matrix at E0(WDH) is
|
JWDH(E0(WDH))=(JW(E0(W))00∗JD(E0(D))0∗∗JH(E0(H))).
|
If R0(WW)<1, R0(DD)<1 and R0(H)<1, all of the real parts of eigenvalues of JWDH(E0(WDH)) are negative, then we get the disease-free equilibrium E0(WDH) in system (2) is stable (Theorem 2.1, Theorem 2.2).
Next we prove the existence of epidemic equilibrium E∗(WDH) and the stability of E∗(WDH).
In (16), the epidemic equilibrium E∗(WDH)=(ˆSW,ˆIW,ˆSD,ˆID,ˆSHH,ˆIHH,ˆSLH,ˆILH). satisfies
|
AHH−βWHˆIWˆSHH−βDHˆIDˆSHH−βHH(ˆIHH+ˆILH)ˆSHH−μHˆSHH = 0
|
(17)
|
|
βWHˆIWˆSHH+βDHˆIDˆSHH+βHH(ˆIHH+ˆILH)ˆSHH−γHˆIHH−αHˆIHH−μHˆIHH = 0
|
(18)
|
|
ALH−βHH(ˆIHH+ˆILH)ˆSLH−μHˆSLH = 0
|
(19)
|
|
βHH(ˆIHH+ˆILH)ˆSLH−γHˆILH−αHˆILH−μHˆILH = 0
|
(20)
|
From (17) + (19), (18) + (20), we get
|
AHH+ALH−βWHˆIWˆSHH−βDHˆIDˆSHH−βHH(ˆIHH+ˆILH)(ˆSHH+ˆSLH)−μH(ˆSHH+ˆSLH) = 0
|
(21)
|
|
βWHˆIWˆSHH+βDHˆIDˆSHH+βHH(ˆIHH+ˆILH)(ˆSHH+ˆSLH)−(γH+αH+μH)(ˆIHH + ˆILH) = 0
|
(22)
|
It is assumed that ηS=ˆSHHˆSHH+ˆSLH ˆSH=ˆSHH+ˆSLH and ˆIH=ˆIHH+ˆILH with ηS∈(0,1).
Then we have
|
AHH+ALH−ηSβWHˆIWˆSH−ηSβDHˆIDˆSH−βHHˆIHˆSH−μHˆSH = 0
|
(23)
|
|
ηSβWHˆIWˆSH+ηSβDHˆIDˆSH+βHHˆIHˆSH−(γH+αH+μH)ˆIH = 0
|
(24)
|
From (23), (24), we can get
|
ˆIH = ηSβWHˆIWˆSH+ηSβDHˆIDˆSHγH+αH+μH−βHHˆSH
|
and
|
ˆSH=12μHβHH[(AHH+ALH)βHH+(γH+αH+μH)(μH+ηSβWHˆIW+ηSβDHˆID)]−12μHβHH[(AHH+ALH)2β2HH+2(AHH+ALH)βHH(γH+αH+μH)(ηSβWHˆIW+ηSβDHˆID−μH)+(γH+αH+μH)2(μH+ηSβWHˆIW+ηSβDHˆID)2]12
|
Similarly to the calculation of Theorem 2.2, we have
|
g2(ˆSH)=μHβHHˆSH2+(AHH+ALH)(μH+γH+αH)−[(AHH+ALH)βHH+(μH+γH+αH)(μH+ηSβWHˆIW+ηSβDHˆID)]ˆSH=0
|
So if R0(WW)>1, there exists epidemic equilibrium E∗(WDH) in (2). The next generation matrix at E∗(WDH) is
|
JWDH(E∗(WDH))=(JW(E∗(W))00∗JD(E∗(D))0∗∗JH(E∗(H)))
|
with
|
JH(E∗(H))=(J11−βHHˆSHH0−βHHˆSHHJ21J220βHHˆSHH0−βHHˆSLHJ33−βHHˆSLH0βHHˆSLHβHH(ˆIHH+ˆILH)J44)
|
|
J11=−βWHˆIW−βDHˆID−βHH(ˆILH+ˆILH)−μH
|
|
J21=βWHˆIW+βDHˆID+βHH(ˆILH+ˆILH)
|
The characteristic equation of JH(E∗(H)) is
|
fH2(λ)=λ4 + a1λ3+a2λ2 + a3λ + a4=0
|
with
|
a1=AHHˆSHH+ALHˆSLH−βHH(ˆSHH+ˆSLH)+2(γH+αH+μH),
|
|
a2=(AHHˆSHH+ALHˆSLH)(γH+αH+μH)−μHβHH(ˆSHH+ˆSLH)−β2HHˆSHHˆSLH+(AHHˆSHH+βWHˆIWˆIHHˆSHH+βDHˆIDˆIHHˆSHH+βHHˆILHˆIHHˆSHH)(ALHˆSLH+βHHˆIHHˆILHˆSLH),
|
|
a3=ALHˆSLH(AHHˆSHH+γH+αH+μH)(γH+αH+μH)−μHβHHˆSLH(AHHˆSHH+γH+αH+μH)−βHHˆSHHALHˆSLH(γH+αH+μH)+AHHˆSHH(ALHˆSLH+γH+αH+μH)(γH+αH+μH)−μHβHHˆSHH(ALHˆSLH+γH+αH+μH)−βHHˆSLHAHHˆSHH(γH+αH+μH),
|
|
a4=AHHˆSHHALHˆSLH(γH+αH+μH)2−μHβHH(ˆSHHALHˆSLH+ˆSLHAHHˆSHH)(γH+αH+μH)
|
It is assumed that μH≈0 for μH is much smaller than other parameters. Then we get a1>0 a2>0 a3>0 a4>0 b1=a1a2−a3a1>0 and c1=b1a3−a1a4b1>0. So if E∗(WDH) exists, all of the real parts of eigenvalues of JH(E∗(H)) are negative according to Routh−Hurwitz stability criterion.
In conclusion, if R0(WW)=AWβWWμW(μW+γW+αW)>1, the real parts of eigenvalues of JWDH(E∗(WDH)) are negative and E∗(WDH) is stable.
From Theorem 2.1, Theorem 2.2 and Theorem 2.3, it is more difficult to satisfy the conditions to control emerging zoonoses with the number of susceptible species increasing. But if there was an epidemic in wildlife with R0(WW)=AWβWWμW(μW+γW+αW)>1, emerging zoonoses might be prevalent in humans.
Next we take Strategy 1, Strategy 2 and Strategy 3 into consideration in order to compare the effects of different isolation and slaughter strategies in wildlife, domestic animals and humans on emerging zoonoses.
Strategy 1.
It is assumed that δ=δS=δI=δR with same slaughter rate in susceptibles, infectives, and recovered individuals in wildlife in order to simply the model (3).
|
{˙SW = AW−βWWIWSW−μWSW−δSW,˙IW=βWWIWSW−μWIW−γWIW−αWIW−δIW,˙RW=γWIW−μWRW−δRW,˙SD = AD−(1−θD)βWDIWSD−βDDIDSD−μDSD,˙ID=(1−θD)βWDIWSD+βDDIDSD−γDID−αDID−μDID,˙RD=γDID−μDRD,˙SHH = AHH−(1−θH)βWHIWSHH−βDHIDSHH−βHH(IHH +ILH)SHH−μHSHH,˙IHH=(1−θH)βWHIWSHH+βDHIDSHH+βHH(IHH+ILH)SHH −γHIHH−αHIHH−μHIHH,˙RHH=γHIHH−μHRHH,˙SLH = ALH−βHH(IHH+ILH)SLH−μHSLH,˙ILH=βHH(IHH+ILH)SLH−γHILH−αHILH−μHILH,˙RLH=γHILH−μHRLH.
|
(25)
|
In (25), we get the control reproductive number in wildlife is R1(WW)=AWβWW(μW+δ)1(μW+γW+αW+δ), the control reproductive number in domestic animals is R1(DD)=ADβDDμD(μD+γD+αD), the control reproductive number in humans is R1(H)=(AHH+ALH)(μH+γH+αH)βHHμH. The epidemic equilibrium of IW, ID and IH are ˆI1W=1βWW(AWβWWμW+δ+γW+αW−μW−δ), ˆI1D = (1−θD)βWDˆI1WˆS1DμD+γD+αD−βDDˆS1D and ˆI1H = (1−θH)ηSβWHˆI1WˆS1H+ηSβDHˆI1DˆS1HγH+αH+μH−βHHˆS1H in strategy 1.
For the epidemic equilibrium of SW, SD and SH, we get ˆS1W=μW+γW+αW+δβW>ˆSW, ˆS1D>ˆSD and ˆS1H>ˆSH for g1(ˆS1D)<0 and g2(ˆS1H)<0.
Theorem 2.4. If R1(WW)<1, R1(DD)<1 and R1(H)<1, the disease-free equilibrium E10(WDH) in system (25) is stable. If R1(WW)>1, there exists epidemic equilibrium E∗∗(WDH), and E∗∗(WDH) is stable.
Strategy 2.
In (4), we get the control reproductive number in wildlife is R2(WW)=AWμWβWW(μW+γW+αW), the control reproductive number in domestic animals is R2(DD)=βDD(μD+γD+αD+ΔI)ADμD, the control reproductive number in humans is R2(H)=βHHμH(AHH+ALH)(μH+γH+αH). The epidemic equilibrium of IW, ID and IH are ˆI2W=1βWW(−μW+AWβWWμW+γW+αW)=ˆIW, ˆI2D = βWDˆI2WˆS2DμD+γD+αD+ΔI−βDDˆS2D and ˆI2H = 1γH+αH+μH−βHHˆS2H(ηSβWHˆI2WˆS2H+(1−ΘH)ηSβDHˆI2DˆS2H) in strategy 2.
For the epidemic equilibrium of SW, SD and SH, we get ˆS2W=μW+γW+αWβW=ˆSW, ˆS2D>ˆSD and ˆS2H>ˆSH for g2(ˆS2H)<0.
In fact, ˆS2D is the smaller root of
|
g3(ˆS2D)=μDβDD(ˆS2D)2−[ADβDD+(μD+γD+αD+ΔI)(μD+βWDˆIW)]ˆS2D+AD(μD+γD+αD+ΔI)=0
|
So we have
|
g3(ˆSD)=μDβDDˆSD2−[ADβDD+(μD+γD+αD+ΔI)(μD+βWDˆIW)]ˆSD+AD(μD+γD+αD+ΔI)
|
If
|
g1(ˆSD)=μDβDDˆSD2−[ADβDD+(μD+γD+αD)(μD+βWDˆIW)]ˆSD+AD(μD+γD+αD)=0
|
and
|
AD−βWDˆIWˆSD−βDDˆIDˆSD−μDˆSD = 0,
|
we get
|
g3(ˆSD)=[ADβDD+(μD+γD+αD)(μD+βWDˆIW)]ˆSD−AD(μD+γD+αD)−[ADβDD+(μD+γD+αD+ΔI)(μD+βWDˆIW)]ˆSD+AD(μD+γD+αD+ΔI)=−ΔI(μD+βWDˆIW)ˆSD+ΔIAD=ΔIβDDˆIDˆSD>0.
|
Then we get ˆS2D>ˆSD with μDβDD>0.
Theorem 2.5. If R2(WW)<1, R2(DD)<1 and R2(H)<1, the disease-free equilibrium E20(WDH) in system (4) is stable. If R2(WW)>1, there exists epidemic equilibrium E∗∗∗(WDH), and E∗∗∗(WDH) is stable.
Strategy 3.
If we took quarantine and isolation strategies in humans only, the impact of wildlife and domestic animals in human epidemic would be never changed comparing to no strategy. So we select the human epidemic model (26) from (5) for further analysis. At the same time, we choose φ(I)=ρ(IHH+ ILH) to simplify the model.
|
{˙SHH = AHH−βWHIWSHH−βDHIDSHH−βHH(IHH+ILH)SHH μHSHH−ρ(IHH+ ILH)SHH+γH1OHH1,˙OHH1=ρ(IHH+ ILH)SHH−γH1OHH1−μHOHH1,˙IHH=βWHIWSHH+βDHIDSHH+βHH(IHH+ILH)SHH −γHIHH−αHIHH−μHIHH−σIHH,˙OHH2=σIHH−γH2OHH2−μHOHH2,˙SLH = ALH−βHH(IHH+ILH)SLH−μHSLH−ρ(IHH+ ILH)SLH +γH1OLH1,˙OLH1=ρ(IHH+ ILH)SLH−γH1OLH1−μHOLH1,˙ILH=βHH(IHH+ILH)SLH−γHILH−αHILH−μHILH−σILH,˙OLH2=σILH−γH2OLH2−μHOLH2.
|
(26)
|
We get the control reproductive number in humans is:
|
R3(H)=(AHH+ALH)βHHμH(μH+γH+αH+σ)
|
Theorem 2.6. If R0(WW)<1, R0(DD)<1 and R3(H)<1, the disease-free equilibrium E30(WDH) in system (5) is stable. If R0(WW)>1, there exists epidemic equilibrium E∗∗∗∗(WDH), and E∗∗∗∗(WDH) is stable.
Table 1. Impact of different strategies on reproductive numbers.
| Strategies | no strategy | Strategy 1 | Strategy 2 | Strategy 3 |
| Reproductive number in wildlife | R0(WW)=RARB | R1(WW)=RARC | R2(WW)= R0(WW) | R3(WW)=R0(WW) |
| Reproductive number in domestic animals | R0(DD)=RDRE | R1(DD)=R0(DD) | R2(DD)=RDRF | R3(DD)=R0(DD) |
| Reproductive number in humans | R0(H)=RGRH | R1(H)=R0(H) | R2(H)=R0(H) | R3(H)=RGRI |
| RA=AWβWW, RB=μW(μW+γW+αW), |
| RC=(μW+δ)(μW+γW+αW+δ), |
| RD=ADβDD, RE=μD(μD+γD+αD), |
| RF=μD(μD+γD+αD+ΔI), RG=(AHH+ALH)βHH, |
| RH=μH(μH+γH+αH), RI=μH(μH+γH+αH+σ). |
3. Numerical simulation
In this section we take avian influenza epidemic in China as an example to analyze the effects of different strategies on emerging zoonoses. Avian influenza is a kind of zoonoses, which have been prevalent in humans since 150 years ago. Avian influenza virus originated from aquatic birds, and it infected domestic birds by sharing watersheds. Humans can be infected by avian influenza virus via infected domestic birds[11,30,6,9]. But for birds, we cannot get the exact parameters to reflect the virus transmission clearly. So we take some similar data to estimate the process of avian influenza virus transmission approximately (TABLE 2).
Table 2. Parameter definitions and their values for avian influenza in China.
| Parameter | Definitions | Values | Sources |
| AW | birth or immigration rate of wild aquatic birds | 0.137 birds/day | Est. |
| μW | natural mortality rate of wild aquatic birds | 0.000137/day | [33] |
| γW | recovery rate of wild aquatic birds | 0.25/day | Est. |
| αW | disease-induced mortality rate of wild aquatic birds | 0.0025/day | Est. |
| AD | birth or immigration rate of domestic birds | 48.72 birds/day | [33] |
| μD | natural mortality rate of domestic birds | 0.0058/day | [33] |
| γD | recovery rate of domestic birds | 0.25/day | [26] |
| αD | disease-induced mortality rate of domestic birds | 0.0025/day | Est. |
| AHH+ALH | birth or immigration rate of humans | 0.07people/day | [23] |
| μH | natural mortality rate of humans | 0.000035/day | [23] |
| γH | recovery rate of humans | 0.33/day | [26, 31] |
| γH1 | remove rate from isolation compartment to susceptible compartment. | 0.5/day | Est. |
| γH2 | remove rate from isolation compartment to recovery individual compartment. | 0.5/day | Est. |
| αH | disease-induced mortality rate of humans | 0.0033/day | Est. |
| R0(WW) | basic reproductive number of wild aquatic birds | 2 | Est. |
| R0(DD) | basic reproductive number of domestic birds | 2 | Est. |
| R0(H) | basic reproductive number of humans | 1.2 | [26] |
| βWD | per capita incidence rate from wild aquatic birds to domestic birds | 6.15∗10−6 | Est. |
| βWH | per capita incidence rate from wild aquatic birds to humans | 2∗10−5 | Est. |
| βDH | per capita incidence rate from domestic birds to humans | 2∗10−5 | Est. |
The number of domestic birds is 4.2 times more than the number of humans in China [7], so we assume that the number of domestic birds is 8400 and the number of humans is 2000 to simplify the calculation. And it is assumed that there are about 1000 wild aquatic birds for no exact data found. And it is assumed that R0(WD) = 0.1∗R0(DD), R0(WH)=0.1∗R0(H) and R0(DH)=0.1∗R0(H).
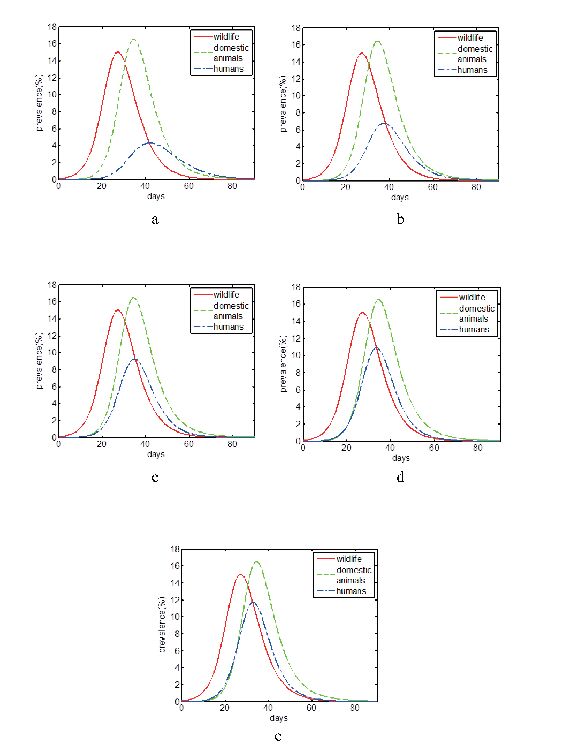
The avian influenza virus transmission has been shown in model (2), which included wildlife, domestic animals, high risk group and low risk group [10,21,13]. For high risk group and low risk group in humans, there may be shown in different proportion in different areas. Less people are needed to take care of live animals in modern farming than tradition. Few people have opportunities to contact with live animals in some areas, which are the potential hosts of some pathogens in emerging zoonoses. But in some other areas, stock raising is the main economy origin of the residents. More people have to look after live animals to help support the family. The proportion of high risk group and low risk group is higher in these areas than others. Here we choose different proportions of high risk group and low risk group, such as 1:9, 1:3, 1:1, 3:1 and 9:1, to reflect emerging avian influenza prevalence in different areas (FIGURE 2).
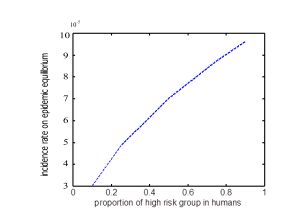
From a to e in FIGURE 2, we get that more and more high proportion of humans are infected in the first 90 days. More people would be infected with higher proportion of them having the opportunity to contact with susceptible animals. From FIGURE 3, we get that the incidence rate on epidemic equilibrium is increasing with higher proportion of high risk group in humans. Although the proportion of high risk group in humans would never change the basic reproductive number, it could impact the final prevalence in humans.
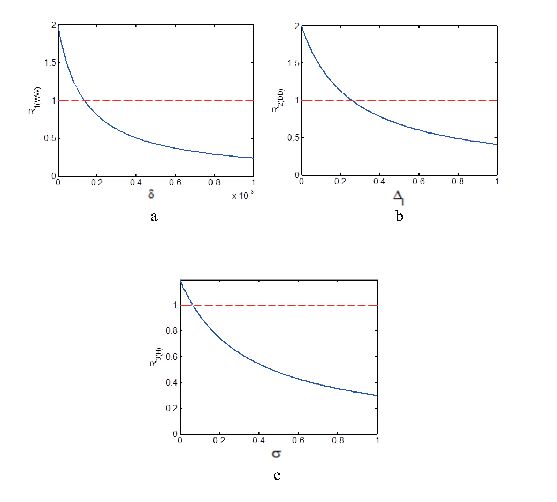
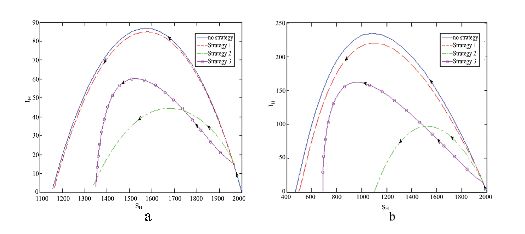
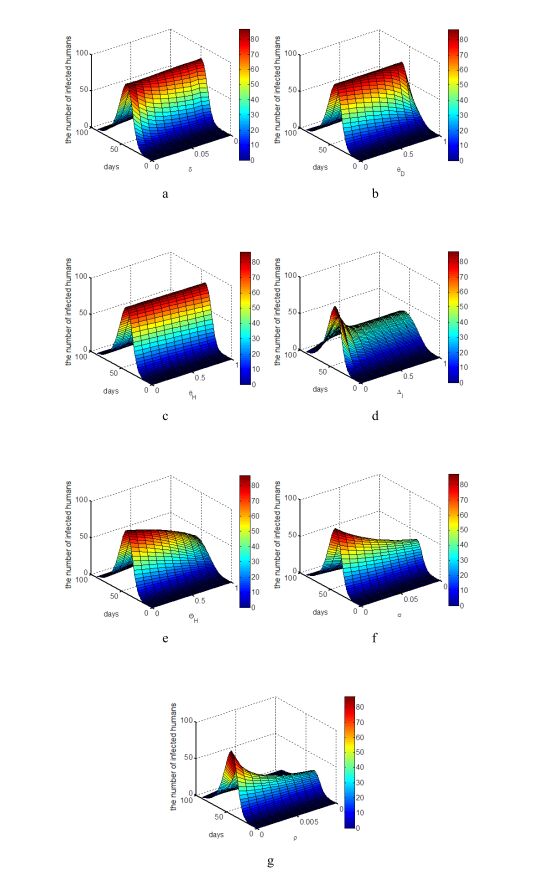
The effects of parameters δ,ΔI,σ in Strategy 1, Strategy 2 and Strategy 3 on control reproductive numbers have been shown in FIGURE 4. The existence of parameters δ,ΔI, and σ would decrease the value of R1(WW),R2(DD) and R3(H). If δ<0.142∗10−3,ΔI<0.258 and σ<0.066,R1(WW),R2(DD) and R3(H) would get the value below threshold to control the zoonoses in wildlife, domestic animals and humans respectively. The effects of Strategy 1, Strategy 2 and Strategy 3 in different areas are shown in FIGURE 5, when IDC=IWC=IHC=15. The effects of δ,θD,θH,ΔI,ΘH,σ and ρ on the number of infected humans in the first 90 days are shown in FIGURE 6.
4. Discussion
From Ebola, Hendra, Marburg, SARS to H1N1, H7N9, more and more zoonotic pathogens come into humans. Tens of thousands of people have dead of these zoonoses in the last hundreds of years. Some public health policies have to be established to answer emerging or remerging zoonoses. For different species participating in an emerging zoonosis, different strategies should been taken for controlling. In this paper, we established model (3), model (4) and model (5) to reflect the effects of Strategy 1, Strategy 2 and Strategy3 about isolation and slaughter in emerging zoonoses respectively. Strategy 1 is the controlling measure for wildlife. Strategy 2 is the controlling measure for domestic animals. And Strategy 3 is the controlling measure for humans.
All of the three strategies would change the basic reproductive number to their own control reproductive number. The involvement of Strategy 1, Strategy 2 and Strategy 3 would change the conditions, which determine the zoonoses prevalence or not. At the same time, we conclude that the extinction of zoonoses must satisfy the conditions ensuring all of basic (control) reproductive numbers in different species are less than 1, whether it is taken controlling strategy or not. But if and only if basic (control) reproductive numbers in wildlife is more than 1, the zoonoses might be prevalent in all of the susceptible species.
The stability analysis on models in section 2 reflects the effects of three strategies on control reproductive numbers and equilibriums. In section 3, some numerical simulations show the effects of the three strategies on avian influenza epidemic in different areas in China at beginning. In this paper, we take isolation and slaughter strategies into consideration to study their effects on emerging zoonoses. But the other effective strategies like vaccination are neglected, which could be proposed in a forthcoming paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (11371048).









 DownLoad:
DownLoad: