Endophytic microorganisms have a wide genetic diversity, being very useful for agriculture, for producing growth regulators and making nutrients available to plants. In this context, this research aimed to isolate and characterize endophytic bacteria from bamboo Guadua sp., in order to evaluate the use of these microorganisms in promoting plant growth in yam plants (Dioscorea rotundata Poir). For this, the macromorphological characteristics of bacterial colonies were studied: shape, borders, consistency, color, brightness. Micromorphological analysis was performed using the Gram method. Biological tests of phosphate solubilization, biological nitrogen fixation and indole acetic acid production were carried out, where the bacteria with the best results were selected to assess the potential to promote the growth of white yam plants. The growth experiment consisted of four treatments: a control (without inoculation) and three treatments with endophytic bacteria, inoculated in pre-acclimated yam plants. Each treatment consisted of 6 repetitions with 5 plants per plot, totaling 30 plants per treatment. The colonies showed similar patterns of morphological characteristics for the three collection sites, with characteristics such as "circular" shape, "entire" border, "creamy" consistency, "brown" color and "bright" shine. We observed that 39.1% of the bacteria were able to perform phosphate solubilization, 61.9% performed atmospheric nitrogen fixation and 26.9% performed indole acetic acid synthesis. The results showed that the endophytic bacteria isolated from Guadua sp. did not promote dry matter accumulation of white yam plants after 45 days of cultivation in a nursery.
1.
Introduction
Among the groups of microorganisms, the so-called “endophytes” stand out because they live within the tissues of the host plants. They have wide genetic diversity, becoming very useful for promoting the defense of plants, for producing growth regulators, making nutrients available to the plants through the biological fixation of nitrogen or by the solubilization of inorganic phosphate, replacing inputs in agriculture that often cause damage to human health and the environment [1].
Strains of endophytes selected from native plant species can be reintroduced into plants of agronomic interest, as they are adapted to local edaphoclimatic conditions and are capable of promoting bioprotection against pathogens, resistance to severe conditions of the environment and promoting growth from production of growth regulators and availability of nutrients in the environment for plants [2,3].
Many species of beneficial endophytic bacteria have been found in native bamboo tissues, many of which have recognized potential to promote plant development when inoculated into crops of economic interest. Liu F et al. [4] identified in bamboo Phyllostachys edulis (Moso bamboo), a large number of endophytic bacteria belonging to the orders Actinomycetales, Rickettiales, Burkholderiales, Enterobacteriales and Rhizobiales. Moshynets OV et al. [5] found in the tissues of bamboos of the genera Phyllostachys spp. and Fargesya spp., 18 bacterial species belonging to the genera Agrobacterium spp, Rhizobium spp, Bacillus spp, Mycobacterium spp, Pseudomonas spp, Paenibacillus spp, Microbacterium spp and Achromobacter spp.
The bamboo species Guadua sp. used in this work are distributed in the southwest of the Brazilian Amazon, occupying mainly temporarily flooded environments. They are characterized by rapid growth and great agammaessiveness in the occupation of understory in open forests [6].
The white yam (Dioscorea rotundata Poir.) belongs to the family Dioscoreaceae, form edible tubers being a food known to contain high nutritional value, composed of vitamins of the B complex, carbohydrates, minerals and medicinal properties [7]. Its economic importance is focused mainly on subsistence agriculture, being managed by both traditional and indigenous populations [8]. Nowadays several species of the genus Dioscorea are recognized, in all of them found in tropical regions [9,10].
Ouyabe M et al. [11] isolated 47 nitrogen fixing bacteria from yams, and identified the isolates within the phylos Proteobacteria, Firmicutes, Actinobacteria, with a large number of genera, the main species corresponding to Brukholderia spp., Bacillus altitudinis, Enterobacter bugandensis.
Despite all the potential of endophytic bacteria to promote biological protection and plant growth in plants, little is known about the biotechnological potential of organisms living in symbiosis in bamboo plants in the Amazon rainforest. Thus, this research aimed to isolate and characterize endophytic bacteria of Guadua sp. in order to test the potential for plant growth promotion in micropropagated white yam (D. rotundata) plants.
2.
Materials and method
2.1. Bamboo collection
The collection of Guadua sp. bamboo for isolation of endophytic bacteria was carried out at the beginning of the rainy season, in the months of December, February and May, in three locations in the southwest of the Brazilian Amazon: Parque Zoobotânico, Sena Madureira and Lago do Silêncio. Parque Zoobotânico is the zoobotanical park of the Federal University of Acre (UFAC) (9°57′24.3″ S 67°52′19.7″ W), and is located in an urban region of the capital Rio Branco (State of Acre). The municipality of Sena Madureira is located east of the capital Rio Branco, in the state of Acre (09°02′10.4″ S 68°47′47.3″ W). The locality Lago do Silêncio is located in the interior state of Amazonas (8°51′41.5″ S 68°42′19.2″ W).
2.2. Preparation of bamboo samples
The leaves and stems obtained from ten individuals per site, which were stored in a thermal box, were used and properly identified, and later taken to the Microbiology Laboratory of the Federal University of Acre for processing of the samples.
In the disinfestation process for the isolation of bamboo endophytic bacteria, the leaf and stem samples were washed with running water to remove debris and dust, and then cut into smaller pieces.
Then, inside the laminar flow chamber, disinfestation was performed according to the method described by Araújo et al. [12], initially with immersion of the fragments in 70% alcohol for 1 minute, 2.5% sodium hypochlorite for 3 minutes, 70% alcohol for 30 seconds, followed by washing in sterile water twice. Then 200 µL of the last wash water were inoculated in the same culture media to control the disinfection process.
After disinfestation, the leaves and stems were cut into 5 mm × 5 mm fragments for sowing on growing medium, then five fragments of plant material per petri dish were sown and one petri dish was used for each growing medium.
2.3. Bamboo endophytes isolation
The culture media used for the isolation of bamboo endophytes were: Soya Triptone broth (STB) with vegetable extract prepared with bamboo, and STB without extract; the other medium used was Luria-Bertani (LB) with vegetable extract of bamboo, and LB half without extract.
The vegetable extract was obtained by grinding 100 g of bamboo leaf into 1000 mL of distilled water, filtered on filter paper, and mixed with the media for preparing the growing medium. To avoid contamination of the media by fungi, 50 µg/mL of the antifungal Cercobin 700 was added at the time of preparation of the culture medium. 15 grams of agar were added to the culture media, then autoclaved at 121 ℃ to 1 atm.
The colonies were purified by the stretch marks method by depletion in culture medium STB 10%. The bacteria were then cryopreserved to maintain their genetic characteristics, transferred to tubes containing liquid STB medium supplemented with 20% glycerol and stored in ultra-freezer at 85 ℃.
2.4. Macro-morphological, micro-morphological and genetic characterization of endophytic bacteria in bamboo
The morphological evaluation was done by the analysis of macro and micro-morphological characteristics. For the macro-morphological analysis of the bacteria, the following characteristics were considered: size (punctiform = less than 1.0 mm), shape (circular, irregular or rhizoid), edges (smooth, lobulated, fringed, wavy), consistency (creamy, viscous, granular or dry), coloration (yellow, pink, white, brown, orange), brightness (bright, opaque), according to the method described by Ribeiro MC [13].
The micro-morphological analysis was based on the Gram method. Bacterial samples were taken with the aid of the bacteriological loop, and mixed with a drop of saline solution on a glass slide.
The staining was done by adding a violet crystal solution for 1 min, then the lugol dye for 1 min, 95% ethyl alcohol for 1 min and safranin for 1 min, washing between each reagent with running water. After staining and drying the slides, they were observed under the common optical microscope to characterize the shape and arrangement of the bacteria.
The molecular identification of bacterial isolates was done by Neoprospecta microbiomes Technologies and the laboratory of the Ministry of Agriculture and Livestock (MAPA). The 1657 isolate was characterized using the preserved sequence of the 16S rRNA gene by the method of enzymatic DNA sequencing based on the termination of dideoxynucleotides analogues [14]. The genomic DNA was submitted to PCR to amplify the DNA. For the study, the initiator oligonucleotides used were PA-AGA GTT TGA TCC TGG CTC AG e PH-AAG GAG GTG ATC CAG CCG CA [15].
The obtained sequences were analyzed and edited in Bioedit 7.0.9.1, aligned to the region sequences (ITS1-5.8S-ITS2), using the ClustalW program, deposited in the GenBank databases, and calculating the species similarity index with the BLASTn. After identifying the species of interest, a phylogenetic tree was built using the neighbor joining method, using the MEGA 7.0 software [16].
Partial sequences of the 16S rRNA gene were used for the identification of isolates 1691 and 1745, with the preparation of the library using PCR (polymerase chain reaction), followed by high throughput Illumina sequencing, and deposited in the NCBI database, with Genebank access number.
The library preparation method was performed by comparing the standard amplification of the 16S rRNA region V4 to its optimized V3-V4 region. In the first PCR reaction (polymerase chain reaction) a pair of 341 F-806 R primers from region V3-V4 was used, because this primer has wide taxonomic coverage in bacteria and archae [17].
The PCR protocol, using V3-V4 primers, followed the conditions: the first PCR primers contain the Illumina sequences based on the TruSeq structure adapter (Illumina, San Diego, CA), allowing for a second PCR with indexing sequences.
The PCR reactions were performed in triplicate, using Taq Platinum (Invitrogene, USA) with the following steps for PCR1 and PCR2. In the PCR1 reaction the samples are submitted to 95 ℃ for 5min, 25 cycles of 95 ℃ for 45 s, 55 ℃ for 30 s, 72 ℃ for 2 min.
In the next PCR2 reaction, the conditions were 95 ℃ for 5 min, 10 cycles of 95 ℃ for 45 s, 66 ℃ for 30 s and 72 ℃ for 2 min. The final PCR reaction was purified using AMPure Xp beads (Beckman Coulter, Brea, CA) and samples were grouped in sequencing libraries for quantification. The amplicom groups were estimated using Picogreen ds DNA assays (Invitrogene, USA), and thus the library groups were diluted to measure the qPCR quantification using the KAPA LIBRARY quantification kit for Illumina platforms (KAPA Biosystems, Woburn, MA).
The libraries were sequenced in MiSeq System, using standard Illumina primer from the Kit. After the sequencing, the bioinformatics conductors did the demultiplexing of the sequence, the adapter and the primer. The readings were normalized to 283 bp [18].
2.5. Physiological characterization of endophytic bacteria in bamboo
2.5.1. Phosphate solubilization test
The bacterial strains isolated have been inoculated in inorganic phosphate medium, which is based on the addition of insoluble phosphate to the medium, which becomes cloudy. The culture medium used was prepared with the following compounds: glucose, 10 g; NH4Cl, 5 g; NaCl, 1 g; MgSO4·7H2O 1g; CaHPO4, 0.8 g; agar, 15 g. After, the volume was adjusted to 1000 mL, and the pH of the medium corrected to 7.2, with the aid of peagameter and solutions of NaOH and HCl 1N.
Four points were inoculated into each petri dish by bacterial strain, then incubated at 28 ℃ for 72 hours. In the tests performed in this work, the presence/absence of bacterial growth film with the formation of halo around the colonies was considered.
To calculate the solubilization index (SI), the diameters of the colonies and the size of the halos were measured, and after the relationship between the diameters of the decomposition halo and the colony were made.
The characterization of the solubilization capacity was carried out according to the method proposed by Berraquero FR [19], based on the SI, where isolated were classified as: low solubilization capacity (SI up to 2), medium solubilization capacity (SI from 3 to 4) and high solubilization capacity (SI > 4).
The results of the solubilization indices obtained were submitted to analysis of variance, using the SISVAR program [20].
2.5.2. Biological nitrogen fixation test
The ability of bacteria to fix atmospheric nitrogen was evaluated by the growth method in test tubes containing 10 mL of semi-solid culture medium (NFb), with the following composition: malic acid, 5 g/L; K2HPO4, 0.5 g/L; MgSO4·7H2O, 0.2 g/L; NaCl, 0.1 g/L; CaCl2·2H2O, 0.02 g/L; KOH, 4.5 g/L; micronutrient solution, 2 mL/L; bromothymol blue solution (0.5% in 0.2 KOH), 2 mL/L; FeEDTA solution (1.64% solution), 4 mL/L; and vitamin solution, 1mL/L; KOH 4.5 g/L; agar, 1.8 g/L. The volume was adjusted 1000 mL, and the pH of the medium corrected to 6.8 with the aid of peagameter and solutions of NaOH and HCl 1 N.
In order to detect nitrogen fixation activity, the bacteria were incubated at 28 ℃ for 72 hours to later observe the presence / absence of growth film in the culture medium [21] and change the color of the medium from green to blue [22].
2.5.3. Indol-acetic acid production test
The bacteria were tested for their indol-acetic acid (IAA) production capacity, grown on 10% STB medium containing L-tryptophan (5 mM), and incubated in the dark at 28 ℃ for four days. The medium was then centrifuged for 5 min at 12,000 rpm, and 900 μL of the supernatant was collected and deposited in tubes to which 400 μL of the Salkowki Reagent were added, and incubated for 30 min at room temperature.
After this period, spectrophotometer readings were taken with a wavelength of 520 nm. The change in color of the medium from transparent to pink indicates that there was production of indol-acetic acid.
2.5.4. White yam growth promotion test
To study the growth promoting effect on white yam plants, three bacterial strains were selected which showed the best results obtained in phosphate solubilization tests, biological nitrogen fixation and indol-acetic acid (IAA) production.
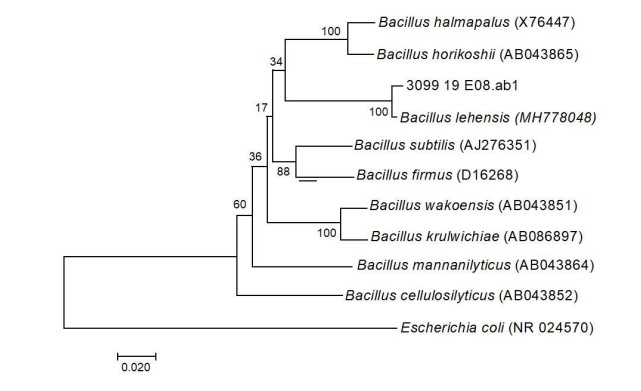
The selected isolates were identified by molecular methods such as Bacillus lehensis, with 100% similarity (recorded in the genebank MT367722), and two other isolates (1691 and 1745), which were classified within the Enterobacteriaceae family (recorded in the genebank MT774770 and MT823305).
These bacteria were reactivated in petri dishes, in 10 mL of solid LB medium, incubated for 48 hours at 28 ℃ in B.O.D. They were then transferred to 500 mL of liquid LB medium in Falcon tubes and incubated for 24 hours at 28 ℃. After this period, the medium was centrifuged to obtain the bacteria precipitate, which was diluted in sterile distilled water with the aid of a spectrophotometer until the concentration of 108 CFU/mL (OD = 0.1; 550 nm).
Yam plants from the micro-propagation were sprayed with the endophytic bacteria solution, and another 30 mL of the bacterial solution was added to the substrate in each beaker (500 mL).
The plants were obtained through in vitro micro-propagation and were pre-acclimatized in the plant tissue culture laboratory of the Federal University of Acre, at a temperature of 25 ℃ for 15 days taken. After this period, they stayed for 30 days in the greenhouse conditions with 50% shade, with irrigation for the first 15 days, and later, for 8 min, four times a day.
For growth and wet mass evaluations, the plants were removed from the pots and washed in running water to remove the substrate adhered to the roots. Height (cm) measurements were performed, using a millimeter scale ruler, and counting the number of leaves. The plants were then cut into three parts to obtain the wet mass (g) of the root, aerial part and tuber.
In the dry mass evaluations, each part of the plant was separated and packed in a paper bag, previously identified with the numbering of each individual, and then submitted to drying in an oven with forced air circulation, at a temperature of 65 ± 3 ℃, in three cycles of 72 hours, until the mass stabilization. All mass measurements were carried out with the aid of a precision analytical balance.
The experimental design for growth analysis was a completely randomized design, with 4 treatments (one control - without inoculation, and three treatments with bacteria - Bac 01, Bac 02 and Bac 03), six replicates and 5 plants per plot. The experimental unit consisted of containers with a single plant each.
The data obtained was submitted to analysis of variance and the comparison of means was performed by the Tukey test at 5% probability, using the statistical program SISVAR [20].
3.
Results and discussions
3.1. Growth and macro-morphological characteristics of bacteria in culture media
Macro-morphological characterization has been reported as an important parameter for the initial identification of colonies of microorganisms, since it enables the first selection of colonies for studies of genetic identification [23], and for other works, such as physiological activity and production of secondary metabolites by these microorganisms.
In this work 289 endophytic bacteria were isolated from Guadua weberbaueri Pilg. and Guadua chaparensis Londoño & Zurita bamboo plants, in the three collection sites. It was observed that the isolates grew in the four types of media used, with most of the bacteria growing fast, between 24 and 48 hours, but some isolates with slow growth (fastidious) were observed, which appeared after a week of cultivation.
The macro-morphological classification of the bacteria colonies was carried out considering the characteristics observed with the naked eye, such as the consistency, coloration, border, shape and brightness of the colonies (Figure 1), revealing a wide diversity of bacteria in the tissues of bamboo.
Anjanadevi IP et al. [24] described the morphology of bacteria, finding large, elevated and opaque irregular colonies as well as small and slightly elevated round colonies, these isolates being later molecularly characterized as Bacillus cereus and Pseudomonas aeroginosas respectively. Bosshard PP et al. [25] concluded that the 16S rRNA gene sequence analysis was the best method for bacteria identification compared to the usual phenotypic methods.
The morphological characteristics of the bacterial colonies were analyzed considering their compositions for each collection site, where the colonies presented similar patterns of morphological characteristics for the three sites, with predominance of the “creamy” Consistency, “brown” Coloration, “shiny” Shining, “circular” Format and “whole” Edge (Table 1).
Some macro-morphological characteristics were obtained exclusively from a single site, indicating differences in the diversity of bacteria colonizing the bamboo tissues in relation to the collection site. Colonies with “pink” coloration were found only in Lago do Silêncio, “orange” and “translucent” coloration only in the Parque Zoobotânico and the “viscous” consistency only found in Sena Madureira.
3.2. Micro-morphological characterization of bacterial isolates
The evaluation and characterization of bacterial isolates by the Gram staining method was performed, as to the forms and arrangements, relating them to the culture media. Most bacteria were characterized as Gram-positive.
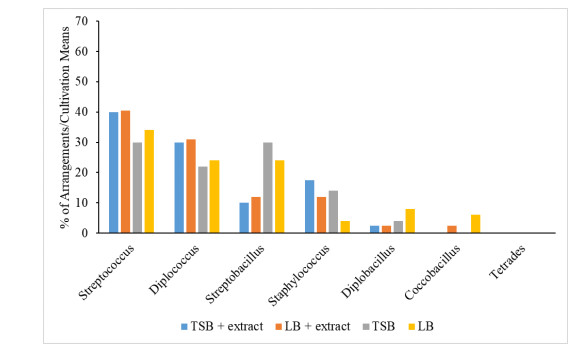
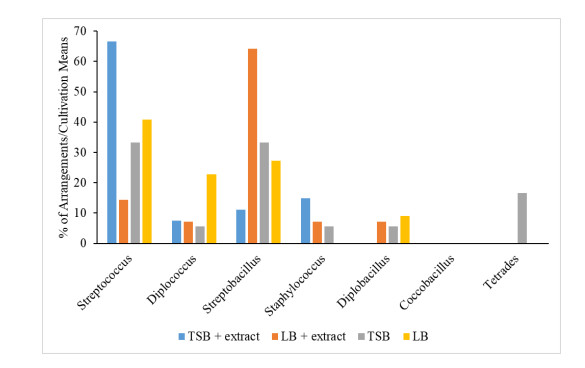
When the plant tissues represented by stems and leaves were considered for the isolation of endophytes, it was possible to observe differences between the compositions of bacterial arrangements in the four types of media. No “tetrade-type” arrangements were found in the stem fragments (Figure 2). On the other hand, no “Coccobacilus” type arrangements were found in the leaf fragments (Figure 3).
Streptococcal isolates were observed to be more abundant in both leaf and stem tissue. The media compositions also influenced the growth of colonies, where TSB with extract showed better results for Streptococcus and Diplococcus resulting from stems, but more representative in foliar tissues. The LB medium added with extract stood out in the growth of Streptobacillus obtained from leaves, being also representative in the growth of Streptococcus and Diplococcus obtained from kaolinitic tissues.
Previous works highlight the group of bacilli and staphylococci as endophytic bacteria of great importance in agriculture, with capacity to promote plant growth [26,27]. Surette MA et al. [28] studied the endophytic bacteria of Daucus carota L. (carrot), and also identified bacteria of the genus Staphylococcus with endophytic characteristic and growth promoting activity.
3.3. Biological tests on endophytic bamboo bacteria
Of the 289 isolates obtained from bamboo plants, 39.1% (n = 113) showed phosphate solubilization activity, 61.9% (n = 179) carried out atmospheric nitrogen fixation and 26.9% (n = 78) were able to produce IAA in culture medium. The data were crossed and only bacteria capable of performing the three activities simultaneously were used for molecular identification and for the subsequent yam plant growth experiment.
Three isolates (1657, 1745 and 1691) were selected by the phosphate solubilization test, that is, those with better solubilization index, and which were able to grow in the nitrogen-free medium and produce indole-acetic acid. In addition, only those isolates that grew again in the LB culture medium to reactivate the bacteria for later identification by molecular methods, were used for the growth promotion experiment.
3.3.1. Inorganic phosphate solubilization test
The phosphate solubilization index obtained for the 113 isolates tested varied from 1.07 to 9.62. The isolates that were selected by comparing the three biological tests (1657, 1745 and 1691), presented the average indexes of 8.17, 6.13 and 5.40, respectively, with no significant differences between them, as shown in Table 2.
Andrade LF et al. [1] tested several species of Bacillus sp. for their ability to solubilize phosphate, fix nitrogen and produce indol-acetic acid, and verified average solubilization rate ranging from 1.03 (Bacillus subtilis) to 3.56 (Lysinibacillus sp.). These indexes were lower when compared with the solubilization indexes found in our work, which found the species Bacillus lehensis (1657 isolate) with a solubilization index of 8.17, and the isolates 1745 and 1691, belonging to the Enterobacteriaceae family, with 6.13 and 5.40 respectively.
These results are similar to those obtained by Abreu CS [29], who classified Enterobacter sp. and Pantoea sp. as potential solubilizers due to the high rates of inorganic phosphate solubilizing activity.
Souza AS et al. [30] also highlight the genera Burkholderia, enterobacter and pantoea as inorganic phosphate solubilizers.
3.3.2. Biological fixation test of atmospheric nitrogen
The biological nitrogen fixation (BNF) by microorganisms is extremely desirable in commercial crops, since nitrogen is a macronutrient needed in large quantities by plants, because this element is part of the structural composition of nucleic acids, proteins and coenzymes [31,32].
The lack of this element can harm the agricultural production of many plant species under stress factors such as climate change, worn out soils, and it is necessary to make available beneficial diazotrophic microorganisms to increase agricultural production, decreasing the use of nitrogen fertilizers, besides contributing significantly to the reduction of the emission of pollutant gases in the atmosphere, such as carbon monoxide, methane and nitrous oxide, from the evaporation of fertilizer residues [33,34].
The results obtained for the biological fixation test of atmospheric nitrogen revealed that 61.9% (n = 179) of the isolates formed film, showing capacity to grow in nitrogen free semi-solid culture medium and, therefore, with potential to be indicated for use in plant growth promotion. Andrade LF et al. [1] found 83% of Bacillus sp. isolates obtained from banana roots being able to grow in nitrogen free culture medium.
The isolate 1657 identified as Bacillus lehensis was able to fix atmospheric nitrogen, and produced indol-acetic acid, and the genus Bacillus sp. is often related to physiological properties favorable to plant growth promotion [35].
Biological nitrogen fixation (BNF) is of great economic value, and diazotrophic endophytic bacteria with such potential are described in the literature, among them isolates belonging to the genus Bacillus [11,36]. Anjanadevi IP et al. [24] classified Bacillus cereus and Pseudomonas aeruginosas, obtained from the yam rhizosphere region, as the best isolates to fix atmospheric nitrogen, Indol-acetic acid (IAA) production, ammonia (NH3) and hydrocyanic acid (HCN).
3.3.3. Indol-acetic acid production test
Indol-acetic acid (IAA) is an auxin naturally produced by superior plants, and can also be produced by endophytic bacteria. It influences the physiology of the plant, promotes its growth, and the development of fruits and roots, contributing to the greater obtaining of water and nutrients by the plant, thus increasing its survival [37,38].
Among the bacteria isolated from Guadua chaparensis and Guadua weberbaueri bamboo plants, 78 isolates (26.9%) synthesized IAA in the culture media used.
3.3.4. Phylogenetic analysis of bamboo bacteria isolates
For the growth promotion test of white yam plants (Dioscorea rotundata), three endophytic bacteria from Guadua sp. bamboo were selected. These bacteria (isolates number 1657, 1691 and 1745), were the ones that obtained the best performance in the tests of phosphate solubilization, biological fixation of nitrogen and production of indol-acetic acid (IAA).
In this work, the bacteria were previously identified by molecular methods, using the sequences of the 16S rRNA gene for phylogenetic analysis, determining that the 1657 isolate belongs to the species Bacillus lehensis with 100% similarity, and registration number in the MT367722 genebank, as shown in Figure 4. The Illumina two-step PCR-based sequencing method grouped isolates 1691 (Genebank MT774770) and 1745 (Genebank MT823305) within the Enterobacteriacea family.
Souza AS et al. [30] verified the diversity of endophytic bacteria in banana trees, and found several species belonging to the genus Bacillus spp. that had genes responsible for promoting plant growth.
3.4. White yam growth promotion experiment (Dioscarea rotundata)
The initial growth promotion experiment of yam plants obtained from in vitro cultivation showed 100% plant survival in all treatments. Three endophytic bacteria obtained from Guadua sp. bamboo, belonging to the following groups, were used for the study of growth promotion in yams Bacillus lehensis and Enterobacteriaceae. These bacteria are commonly found in the internal tissues of various plant species, including yams [8].
After 45 days of cultivation, under nursery conditions, the effect of the three bacteria (isolates 1657, 1691 and 1745) selected by biological tests to promote the growth of micro-propagated white yam plants was evaluated.
It was observed that yam plants showed great vigor, healthy appearance, with wide leaves and green coloration, indicating that the plants received adequate nitrogen source, which can be attributed to the biological activity of the bacteria, which probably enhanced the absorption of nutrients contained in the commercial substrate vivatto plus®.
Regarding the adaptation and survival of yams at 45 days of cultivation in a nursery environment, micro-propagated plants showed 100% survival, indicating that the method of adaptation to nursery conditions was effective in ensuring the survival of the plants, regardless of the inoculation of bacteria for the process.
The bacteria Bacillus lehensis and Enterobacteriacea used in this experiment belong to taxonomic groups described in previous works as endophytic plant growth promoters [11,38]. Souza JT [27] found in the tissues of yams, the species Bacillus cereus living in symbiosis in all organs of this plant, while Bacillus pumilus was present only in the root tissues.
The results found in this work, in which the yam plants were treated with endophytic bacteria of the Enterobacteriaceae family and of the species Bacillus lehensis, showed that in the evaluation after a shorter period of cultivation (45 days), no significant differences were observed between the treatments for the parameters “Height of Aerial Part” and “Number of Leaves”, according to Table 3.
The witness treatment and Bac 02, showed a significantly higher rate of “Wet biomass increment” than the other treatments, with averages of Enterobacteriaceae (Bac 02 and Bac 03) related, addition of fresh biomass during the initial growth phase (45 days) of yam plants grown in a nursery environment.
In studies performed by Peña-Yam et al. [38], covering the complete life cycle of the plant, the effect of bacteria in promoting the growth and production of Capsicum annuum (pepper) was demonstrated by immersing seeds in solutions of Bacillus sp. (108 CFU/mL), resulting in a significant increase in leaf diameter, number of flowers, fresh root biomass and total fresh biomass.
As for the effects of the treatments on the total wet biomass of the plants at the end of the 45-day period of cultivation, a significant influence of the treatments used on the evaluated parameters of “Tubers Wet Mass” (TubWM), “Wet Mass of Roots” (WMR), and “Total Wet Mass” (TOTALWM) was found. The “Wet Air Mass” (WAM), did not show significant difference between treatments. The values obtained in each treatment are presented in Table 4.
Comparisons of the contrasts performed through Tukey’s test for the results of “Total Wet Mass” revealed significantly higher averages for the “witness” and “Bac 02” treatments, when compared with Bac 01 and Bac 03, demonstrating that the physiological activity of the bacteria used in the experiment influenced the production of wet biomass by yam plants.
In this work, the root biomass was influenced by treatments, where the “witness” treatments, Bac 02 and Bac 03 (Enterobacteriaceae), obtained the best averages, not differing significantly from each other. The Bac 01 treatment presented a significantly lower average than the other treatments.
The formation of heavier roots in the early stages of plant growth can ensure better conditions for survival and growth of plants, as the roots fix the plant in the substrate, absorb water and mineral nutrients [39].
The highest values obtained for the “Tubers Wet Mass” were observed in the testimonial treatment, which was significantly higher (p < 0.05), than the other treatments. Since the tuber is the main product of interest to yam plants, research works in general value treatments that guarantee larger and heavier roots and tubers [40].
The growth potential of different parts of yam plants may change over time due to possible physiological adaptations between the plants, micro-organisms and the growing environment [11,39,41].
Palaniyandi AS et al. [42] observed a significant increase in dry mass and number of lateral roots in seedlings of Arabidopsis (peanuts), grown “in vitro”
with added actinobacteria isolated from yam plants, compared to non-inoculated plants, attributing the result of in vitro growth promotion to the production of growth hormones by IAA-producing bacteria.
When comparing averages of the results obtained for the production of dry matter, no significant difference was found between the treatments for “Dry Mass of roots”. The mean dry biomass of the parameters TubDM, DMR, DAM and TOTALDM, produced in each treatment are presented in Table 5.
The evaluations of “Dry Air Mass” and “Total Dry Mass”, showed a tendency of greater accumulation of biomass for the treatment witness with significantly higher averages in relation to the other treatments.
In relation to “Tuber Dry Mass” the witness and Bac 02 treatments presented the best averages, differing statistically (p < 0.05) from the other treatments. The result of Bac 02 indicates that a higher rate of tuber mass accumulation does not necessarily increase the total dry biomass of the plants in relation to the plants of other treatments.
Takada K et al. [43] suggest that water yam (Dioscorea alata L.) grown under unfertilized soil conditions has 38.8% nitrogen absorbed by endophytic microorganisms directly from the air, highlighting the importance of biological fixation of atmospheric nitrogen for the growth of yam plants. This estimate was confirmed by Ouyabe M et al [11] using endophytic nitrogen-fixing bacteria in yams after 60 days of culture.
The interactions between yam plants and endophytic microorganisms providing higher growth increments and biomass indicate the ability of some bacteria to make nutrients available to the plants, a desired condition in field crops intended for agricultural production. Such interactions are determinant and certainly influence the fixing action of nitrogen and phosphate, the production of hormones and other plant growth factors, performed by endophytic microorganisms [39,44].
It should be noted that in plants obtained from in vitro cultivation, the factor “time of cultivation” must be taken into account so that an efficient interaction between the plant, environment and microorganisms occurs, so that the plant acquires the physiological benefits provided by endophytic microorganisms [11].
4.
Conclusion
The data of this research suggest a wide variety of bacterial colonies isolated from bamboo tissues Guadua sp., demonstrated by the different macro and micromorphological characteristics observed, and several bacteria showed high phosphate solubilization activity, nitrogen fixation and production of IAA.
Since the increase in dry mass is the main indicator of growth and mass accumulation by the plant, the data of this work showed that the tested bacteria were not efficient in increasing the dry matter accumulation by the white yam plants, demonstrating that the treatment control was more efficient than treatments with the addition of the endophytic bamboo bacteria Guadua sp. New studies can be carried out to verify the potential of these bacteria in promoting the growth of white yam (Dioscorea rotundata) in stages after adaptation to nursery conditions.
Acknowledgments
We thank the Federal University of Acre (UFAC) and EMBRAPA, in Rio Branco, Acre, Brazil, for their participation for this study.
Conflict of interest
The authors declare no conflict of interest.









 DownLoad:
DownLoad: