Due to the natural, innocuous and bioactive compounds that medicinal plants contain, their application in aquafeeds has great potential to ensure more sustainable aquaculture. The aim of the present study was to evaluate the in vitro biological activities of purslane (Portulaca oleracea L.) as well as its in vivo effects after dietary administration to gilthead seabream (Sparus aurata L.) specimens. To carry out this work we first analysed the in vitro effect purslane aqueous and ethanolic extracts on head-kidney (HK) leucocytes from gilthead seabream, as well as their cytotoxic, bactericidal and antioxidant activities. After that, we studied the effect on gilthead seabream growth performance and immune status after the addiction of purslane to its diet. Regarding the in vitro study, purslane aqueous and ethanolic extracts had immunostimulant properties on HK leucocyte phagocytosis, bactericidal activity against marine pathogenic bacteria (Vibrio harveyi, V. anguillarum and Photobacteium damselae) and cytotoxic (mainly against PLHC-1 tumor cells) and antioxidant activities. On the other hand, the dietary inclusion of 2% purslane for 15 or 30 days had no impact on growth performance while improving the fish's immune status. More specifically, skin mucus IgM levels and the phagocytic capacity of HK leucocytes of fish-fed purslane diets for 15 and 30 days, respectively, increased statistically significant with respect to the values recorded for the fish-fed control diet. Overall, the results suggest that purslane extracts, when used in adequate concentrations, have different beneficial properties, and that the dietary incorporation of purslane improves some immune parameters at local and systemic levels while having no negative effect on growth. These results make purslane an interesting candidate for incorporation as an additive in functional diets for farmed fish.
1.
Introduction
Disease outbreaks represent a significant limiting factor in aquaculture development worldwide. For decades, using antibiotics has been a common practice to curing and preventing diseases in order to improve production performance and to avoid what may be substantial economic losses [1]. However, the European Union has banned the use of several antibiotics [Regulation (EC) No 1831/2003], leading the scientific community to look for natural and sustainable alternatives for antibiotics. Among them, the use of medicinal plants (including herbal products or plant extracts) should be considered. Furthermore, some of these plants have been tested for their possible immunostimulant effects on farmed fish [2,3,4,5,6].
In recent years, notable success has been achieved with herbs as immunostimulants, whether using the entire plant or just some of its components [7,8]. It has been reported that the administration of herbal dietary supplements obtained from more than 60 different medicinal plant species improves growth performance, immune responses and antimicrobial capacity, while stimulating the appetite and the antioxidant system [9] in fish (Van Hai, 2015). Purslane (Portulaca oleracea L.) is a warm-climate plant with a cosmopolitan distribution including tropical and subtropical areas worldwide and can be found in Europe and North Africa, North America, Asia, Australia and New Zealand [10,11,12,13]. Curiously, this plant is classified by WHO as one of the most-used medicinal plants, it has been given the term ‘Global Panacea’ [14] and it is known as a ‘vegetable for long life’ [15]. Purslane has been well-known and used since ancient times in folk and traditional medicine to treat several diseases and disorders [13]. Moreover, this plant is used in the production of food products for human consumption [12,16].
Regarding purslane’s biological activities, this plant presents many, including antimicrobial anticancer, antioxidant, anti-inflammatory, immunomodulatory and wound healing [16], which is due to the presence of abundant and varied secondary metabolites, most of them phenolic compounds such as phenolic acids, flavonoids, terpenes, alkaloids, tannins and coumarins, among many others substances [11,12,16]. In addition, purslane has high potential for being used in human and animal food as well as a pharmacological agent in medicine. However, although several studies have demonstrated its immunostimulatory activity in mice, chicken and human cell lines [17,18,19,20,21], to the best of our knowledge, there are no published studies on purslane’s effects on fish.
Taking into account all these considerations and in order to increase our knowledge of its health-related effects and to confirm the possibility of using it in plant-based diets for farmed fish, we made the present study. Firstly, the in vitro effects of purslane extracts (both aqueous and ethanolic) on gilthead seabream (Sparus aurata L.) head-kidney (HK) leucocyte activities (viability, phagocytosis, respiratory bursts and peroxidase) were evaluated. Furthermore, the possible cytotoxic activity of such extracts on SAF-1 cells (cell line obtained from S. aurata fibroblasts), the PLHC-1 cell line (hepato-carcinoma obtained from Poeciliopsis lucida, topminnow) and the bactericidal activity against three bacterial pathogens for fish (Vibrio harveyi, V. anguillarum and P. damselae) were assessed, and the antioxidant activity of the extracts was determined. Secondly, the effects of dietary supplementation of purslane on gilthead seabream growth performance and immune status were tested. Gilthead seabream was used as a representative species of the marine Mediterranean aquaculture.
2.
Materials and methods
2.1. Purslane extracts
Purslane was collected from the surroundings of the University of Murcia (Espinardo Campus, Spain) and its identification was confirmed using standard methods by researchers of the Botany Department of the University of Murcia (Spain). Stem and leaves were carefully washed with distilled water, dried in an incubator (60ºC for 2 days) and crashed until to be powder with an electric grinder. One g of powder and forty mL of water or absolute ethanol (1:40 w/v) were used for extracts preparation [22,23]. To prepare the aqueous extracts, the powder was macerated and shaken with boiling water (initially) and maintained for 4 h at 25ºC. For the preparation of ethanolic extracts, dry powder was macerated and shaken with pure ethanol (1:40 w/v, 48 h, 25ºC). Prior to use in the assays, the extracts were filtered using nylon net sterile filters of 0.22 mm diameter. Finally, aqueous extracts were then freeze-dried (lyophilized) while ethanolic extracts were evaporated at 40 ºC until dryness and both were stored at −20℃.
2.2. Fish used for in vitro studies
Five specimens (52.75 ± 3.62 g weight) of the hermaphroditic protandrous seawater teleost gilthead seabream (S. aurata L.), obtained from a local farm (Murcia, Spain), were kept in re-circulating seawater aquaria (250 L) in the Marine Fish Facilities at the University of Murcia. The water temperature was maintained at 20 ± 2℃ with a flow rate of 900 L h−1 and 28‰ salinity. The photoperiod was 12 h light: 12 h dark. Fish were allowed to acclimatize for 15 days before starting the trial. Fish were fed with a commercial pellet diet (Skretting, Spain) at a rate of 2% body weight day−1. Before sampling, the fish were starved for 24 h and killed by using an overdose of MS-222 (100 mg mL−1 water, Sandoz). All experimental protocols were approved by the Ethical Committee of the University of Murcia.
2.3. Head-kidney leucocyte isolation and incubation with purslane extracts
Before the dissection of the HK, the specimens were bled as described below. Blood was collected from the caudal vein and afterwards fish were dissected to obtain HK fragments, isolating the leucocytes [24]. Briefly, HK were cut into small fragments and transferred to 12 mL of sRPMI [RPMI-1640 culture medium (Gibco) supplemented with 0.35% sodium chloride (to adjust the medium’s osmolarity to gilthead seabream plasma osmolarity of 353.33 mOs), 3% foetal calf serum (FCS, Gibco), 2 mM L-glutamine (Gibco), 100 i.u. mL-1 penicillin (Flow) and 100 mg mL-1 streptomycin (Flow)]. HK leucocytes were obtained by forcing fragments of the organ through a nylon mesh (mesh size 100 mm), washed twice (400 g, 10 min), counted in an automatic counting chamber (BioRad) and adjusted to 2 × 107 cells mL-1 in sRPMI. Cell viability was determined by the trypan blue exclusion test. All the cellular immune functions were performed only in viable cells.
To study the possible effects of purslane extracts on HK leucocyte viability and immune activities, aliquots of 50 µL of the HK leucocytes suspension were dispensed into glass tubes (Falcon) to ascertain viability and phagocytic activity, 50 µL into a flat-bottomed 96-well plates to assess respiratory burst activity and 5 µL into a flat bottomed 96-well plates for peroxidase activity. Afterwards, same aliquots of aqueous (directly dissolved in sRPMI) or ethanolic extracts [dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich) and then further diluted in sRPMI] were added at a final concentration of 0.001, 0.1, 0.5 and 1 mg mL-1. For controls, sRPMI replaced the aqueous extract and 1% DMSO in sRPMI replaced the ethanolic extracts. Leucocytes were incubated in the presence of the extracts for 24 h at 21ºC in an incubator with 5% CO2 and 85% humidity.
2.4. HK leucocyte viability
After incubation, HK leucocyte viability was studied adding 50 µL of propidium iodide (PI) (400 mg mL-1, Sigma-Aldrich) to each 100 µL aliquot of HK leucocytes (previously incubated with the extracts, as described above). The tubes were gently mixed before analysis in a FACScan flow cytometer (Becton Dickinson) with an argon-ion laser adjusted to 488 nm. Analyses were performed on 5,000 cells, which were acquired at a rate of 300 cells s-1. Data were collected in the form of two-parameter side scatter (granularity, SSC) and forward scatter (size, FSC), and green fluorescence (FL1) and red fluorescence (FL2) dot plots or histograms were made on a computerized system. Dead cells were estimated as the percentage of cells with PI (red-PI fluorescent cells). A quantitative study of the flow cytometric results was made using the statistical option of the Lysis Software Package (Becton Dickinson).
2.5. HK leucocyte immune activities
2.5.1. Phagocytic activity
The phagocytic activity of gilthead seabream HK leucocytes was studied by flow cytometry [25]. Heat killed (30 min, 60ºC) and lyophilized Saccharomyces cerevisiae, strain S288C, were washed twice, counted and adjusted to 108 yeast cells mL-1 in sRPMI. To label yeast cells with fluorescein isothiocyanate (FITC, Sigma-Aldrich) they were incubated with 5 mg mL-1 FITC at 22ºC with constant stirring (40 cycles min-1) and in darkness for 15 min [26]. After labelling, free FITC was removed by washing twice in phosphate buffer saline (PBS) and the yeast cells were resuspended in sRPMI. FITC-labelled yeast cells were acquired for flow cytometric study. The staining uniformity was examined and then the yeast cell suspensions were aliquoted and stored at −80ºC.
Phagocytosis samples consisted of 60 µL of labelled-yeast cells and 100 µL of HK leucocytes (previously incubated as described above). Samples were mixed, centrifuged (400 g, 5 min, and 22℃), resuspended and incubated for 30 min at 22℃. At the end of the incubation time, samples were placed on ice to stop phagocytosis and 400 µL ice-cold PBS was added to each sample. The fluorescence of the extracellular yeasts was quenched by adding 50 µL ice-cold trypan blue (0.5% in PBS). Standard samples of FITC-labelled S. cerevisiae or HK leucocytes were included in each phagocytosis assay. All samples were analysed in a FACScan flow cytometer set to analyse the phagocytic cells, which show the highest SSC and FSC values. Data of 5,000 phagocytic cells were collected and the phagocytic ability, defined as the percentage of phagocytic cells with one or more ingested bacteria (green-FITC fluorescent cells), and phagocytic capacity, defined by their mean fluorescence intensity, equivalent to the relative number of ingested yeast cells per cell, were assessed.
2.5.2. Respiratory burst activity
The respiratory burst activity of HK leucocytes was studied by a chemiluminescence method [27]. Briefly, 100 µL of Hank’s balanced salt solution (HBSS, Gibco) containing 1 mg mL-1 phorbolmyristate acetate (PMA, Sigma-Aldrich) and 10-4 M luminol were added to the 100 µL of HK leucocytes (previously incubated as described above). The plates were shaken and immediately read in a chemiluminometer (BMG, FluoStar Galaxy). Measurements were performed in 30 cycles of 2 min each. The kinetic of the reactions was analysed and the maximum slope of each curve was calculated. Luminescence backgrounds containing HK leucocytes without PMA and luminol were also analysed.
2.5.3. Peroxidase activity
The total peroxidase activity of HK leucocytes was measured according to Quade and Roth [28]. To do this, HK leucocytes (previously incubated as described above) were lysed for 10 min with 0.002% cetyltrimethylammonium bromide (CTAB, Sigma-Aldrich) at 60 rpm. Afterwards, 100 µL of 10 mM 3, 3’, 5, 5’-tetramethylbenzidine hydrochloride (TMB, Sigma-Aldrich) and 5 mM hydrogen peroxide (H2O2) (both substrates prepared daily) were added and after 2 min, 50 µL of 2 M sulphuric acid (H2SO4) were also added to stop the reaction. The absorbance of the samples was measured at 450 nm in a microplate reader. Control samples containing leucocytes without substrates were also analysed. Standard samples without leucocytes were used as blanks.
2.6. Cytotoxicity of purslane extracts on fish cell lines
The established cell line SAF-1 (ECACC nº 00122301) was seeded in 25 cm2 plastic tissue culture flasks (Nunc) in L-15 Leibowitz medium (Life Technologies), supplemented with 10% FCS, 2 mM L-glutamine, 100 i.u. mL-1 penicillin and 100 mg mL-1 streptomycin. Cells were grown at 25ºC in a humidified atmosphere (85% humidity). Exponentially growing cells were detached from culture flasks by brief exposure to trypsin (0.25% in PBS, pH 7.2–7.4), according to the standard trypsinization methods. The detached cells were collected by centrifugation (200 g, 5 min, 25ºC) and cell viability was determined by the trypan blue exclusion test.
The established cell line PLHC-1 (ATCC® CRL2406™) was seeded in 25 cm2 plastic tissue culture flasks in Minimum essential medium (Eagle) with 2 mM L-glutamine and Earle's salts adjusted to contain 1.5 g L-1 sodium bicarbonate, 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate, 5% FCS, 100 i.u. mL-1 penicillin and 100 mg mL-1 streptomycin. Cells were grown at 30ºC in a humidified atmosphere (85% humidity) with 5% CO2. Exponentially growing cells were detached from the culture flasks by brief exposure to of trypsin (0.05% in PBS, pH 7.2–7.4), according to the standard trypsinization methods. The detached cells were collected by centrifugation (200 g, 5 min, 30ºC) and cell viability was determined by the trypan blue exclusion test.
A cytotoxicity assay of each cell type was performed in five replicates at each concentration of each extract. When cell lines were approximately 80% confluent, cells were detached from the flasks with trypsin (as described before), and aliquots of 100 µL containing 50,000 cells well-1 were dispensed into 96-well tissue culture plates and incubated (24 h, at the temperature for each cell line). This cell concentration was previously determined in order to obtain satisfactory absorbance values in the cytotoxic assay and to avoid cell overgrowth. After that, the culture medium was replaced by 100 µL well-1 of the aqueous or ethanolic extracts at 0.001, 0.05, 0.1, 0.25, 0.5, 0.75 and 1 mg mL-1. Control samples received the same volume of culture medium (for the aqueous extracts) or 1% DMSO (for the ethanolic extracts). Cells were incubated for 24 h and then their viability was determined using the MTT assay, which is based on the reduction of the yellow soluble tetrazolium salt (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) (MTT, Sigma-Aldrich) to a blue, insoluble formazan product by mitochondrial succinate dehydrogenase [29,30]. For this, cells were washed with PBS and 200 µL well-1 of MTT (1 mg mL-1) were added. After 4 h of incubation, cells were washed again and the formazan crystals were solubilized with 100 µL well-1 of DMSO. Plates were shaken (5 min, 100 rpm) in dark conditions and the absorbance at 570 nm and 690 nm determined in a microplate reader.
2.7. Bactericidal activity of purslane extracts
Three pathogenic bacteria for fish (V. harveyi, V. anguillarum and P. damselae subsp. piscicida) were used in the bactericidal assays. They were cultured for 48 h at 25ºC in Triptic Soy Agar (TSA, Difco Laboratories), and then single colonies inoculated in Triptic Soy Broth (TSB, Difco Laboratories), both supplemented with NaCl to a final concentration of 1% (w/v), and growth with continuous shaking (100 rpm) for 24 h. Exponentially growing bacteria were washed and resuspended in sterile PBS at 108 colony forming units (c.f.u.) mL-1.
Bactericidal activity was determined following the method of Stevens et al. [31] with some modifications. For this, aliquots of 20 µL of bacteria were added to wells of a flat-bottomed 96-well plate (in six replicates) and incubated with the same volume of aqueous or ethanolic extracts, ranging from 0.001 to 1 mg mL-1, for 5 h at 25ºC. PBS solution was added to some wells instead of the extracts and served as positive control. Then, 25 µL of MTT (1 mg mL-1) were added to each well and the plates were newly incubated for 10 min at 25ºC to allow the formation of formazan. Plates were then centrifuged (2,000 g, 10 min), and the precipitates dissolved in 200 µL of DMSO and transferred to a flat-bottomed 96-well plate. The absorbance of the dissolved formazan was measured at 570 nm. Bactericidal activity was expressed as percentage of non-viable bacteria, calculated as the difference between absorbance of surviving bacteria in test samples compared to the absorbance of bacteria from positive controls (100%).
2.8. Total antioxidant activity (TAA) of purslane extracts
The TAA of aqueous or ethanolic extracts was analysed by the 2, 2’-azino-bis-3(ethylbenzothiazoline-6-sulphonic acid) (ABTS) method [32], which is based on the ability of the antioxidants in the sample to reduce the radical cation of ABTS, as determined by the decolouration of ABTS+, and measuring the quenching of the absorbance at 730 nm. This activity is calculated by comparing the values of the sample with a standard curve of ascorbic acid and expressed as ascorbic acid equivalents (mmol) mg protein-1. Samples of 50 µL of aqueous or ethanolic extracts at 0.1, 0.5 and 1 mg mL-1 were added to 950 µL of ABTS+ and the decrease of absorbance was measured in a spectrophotometer (BOECO S-22 UV/Vis) using as blank of reaction with PBS. The samples were analysed in triplicate. A standard curve was done with ascorbic acid (Sigma-Aldrich) and the antioxidant capacity of aqueous and ethanolic extracts interpolated from the adjusted curve.
2.9. Fish used for in vivo studies
In order to use the fewest possible number of fish to satisfy the 3Rs principle without affecting the possible significant differences between treatments, twenty-four specimens (50.56 ± 1.6 g weight and 14.26 ± 0.17 cm length) of the hermaphroditic protrandous seawater teleost gilthead seabream (S. aurata L.), obtained from a local farm (Murcia, Spain), were maintained as described above.
2.10. Preparation of diets and experimental design
A commercial pellet diet (Skretting, Spain) was crushed and mixed with tap distilled water before adding the correct amount of crushed purslane powder. The feds were pelleted to obtain diets supplemented with 0% (control, non-supplemented diet) or 2% purslane (diet supplemented with 2 g of purslane powder in 100 g of feed). The final products were introduced into a meat grinder at room temperature and pelleted. The resulting pellets were dried in a forced-air oven (37℃, 24 h) before packaging in polypropylene bags and storing at 4℃ until use.
Fish were randomly distributed in four identical tanks (6 fish per tank), where the following groups were established in duplicate: (1) Control, non-supplemented diet (0%), and (2) Purslane diet (2%). The fish were fed at a rate of 2% body weight day-1 for 30 days. Three fish from each tank (six fish from each experimental diet) were sampled after 15 or 30 days. All specimens were starved for 24 h prior to sampling and were killed by using an overdose of MS-222 (Sandoz, 100 mg L-1 water) and sampled.
2.11. Sample collection
Blood samples were collected from the caudal vein with an insulin syringe. Blood samples were left to clot at 4ºC for 4 h, and later the serum was collected after centrifugation (10,000 g, 5 min, 4ºC) and stored at −80ºC until use. HK leucocytes were obtained as described above and adjusted to 107 cells mL-1 in sRPMI to perform all the studies. Skin mucus samples were collected using the method described by Guardiola et al. [33]. Briefly, skin mucus was collected by gentle scraping the dorso-lateral surface of seabream specimens using a cell scraper with sufficient care to avoid contamination with blood and urogenital and intestinal excretions. Collected skin mucus samples were vigorously shaken and then centrifuged (2,000 g, 10 min, 4ºC). The protein concentration in the supernatant of each sample was determined by Bradford’s dye binding method [34] using bovine serum albumin (BSA, Sigma) as the standard.
2.12. Growth parameters
The body weight and length of each fish were measured before the trial and at the beginning of each sampling. Growth was studied by obtaining the initial weight (Wi), final weight (Wf), weight gain (%WG), specific growth rate (%SGR), and condition factor (CF) [35].
2.13. Cellular immune parameters
The phagocytic, respiratory burst and peroxidase activity of 107 mL-1 HK leucocytes were studied as described above.
2.14. Serum and skin mucus humoral immune parameters
2.14.1. Total IgM levels
Total IgM levels were analysed using the enzyme-linked immunosorbent assay (ELISA) [36]. Briefly, 100 µL of serum (diluted 1:500 with 50 mM carbonate-bicarbonate buffer, pH 9.6) or skin mucus (diluted 1:5 diluted with the above buffer) were placed in flat-bottomed 96-well plates in triplicate and coated overnight at 4℃. Samples were rinsed 3 times with PBS-T [20 mM phosphate buffer (PBS) and 0.05% Tween-20, pH 7.3], blocked for 2 h at room temperature with blocking buffer (PBS-T containing 3% bovine serum albumin BSA) and rinsed again. The plates were then incubated for 1 h with 100 µL well-1 of mouse anti-gilthead seabream IgM monoclonal antibody (Aquatic Diagnostics Ltd.) (1:100 in blocking buffer), washed and incubated with the secondary antibody anti-mouse IgG-HRP (1:1,000 in blocking buffer, Sigma). After exhaustive rinsing with PBS-T, the samples were developed using 100 µL of a 0.42 mM solution of 3, 3, 5, 5-tetramethyl benzidine hydrochloride (TMB, Sigma), prepared daily in a 100 mM citric acid/sodium acetate buffer (pH 5.4) containing 0.01% H2O2. The reaction was allowed to proceed for 10 min, stopped by the addition of 50 µL of 2 M H2SO4 and the plates read at 450 nm in a plate reader. Negative controls consisted of samples without serum, skin mucus or primary antibody, whose optical density (OD) values were subtracted for each sample value. Data are presented as the OD at 450 nm for each sample value.
2.14.2. Lysozyme activity
Lysozyme activity was measured according to a turbidimetric method [37] with some modifications. Briefly, 20 μL of serum or skin mucus were placed in flat-bottomed 96-well plates. To each well, 180 µL of freeze-dried Micrococcus lysodeikticus (0.2 mg mL-1, Sigma) in 40 mM sodium phosphate (pH 6.2) was added as lysozyme substrate. As blanks of each sample, 20 μL of serum or skin mucus were added to 180 μL of sodium phosphate buffer. The absorbance at 450 nm was measured after 20 min at 35ºC in a plate reader. The amounts of lysozyme present in serum and skin mucus were obtained from a standard curve made with hen egg white lysozyme (HEWL, Sigma) through serial dilutions in the above buffer.
2.14.3. Peroxidase activity
The peroxidase activity was measured with the same method as described above with some modifications. Briefly, 5 μL of serum and 10 μL of skin mucus were diluted with 45 μL or 40 μL Hank’s buffer (Hank’s Balanced Salt Solution, HBSS) without Ca2+ or Mg2+ in flat-bottomed 96-well plates, respectively. Aliquots of 100 μL of 20 mM TMB and 5 mM H2O2 were then added to each well and serves as substrates. After 2 min the reaction was secured by adding 50 μL of 2 M H2SO4 and the OD was measured at 450 nm in a plate reader. Samples without serum and skin mucus were used as blanks.
2.14.4. Protease activity
Protease activity was measured using the azocasein hydrolysis assay [38] with some modifications. Briefly, 10 µL of serum or 100 µL of skin mucus were incubated overnight at RT and in agitation with 100 µL of 100 mM ammonium bicarbonate buffer and 125 µL of 2% (serum) or 0.7% (skin mucus) azocasein (Sigma-Aldrich) diluted in 100 mM ammonium bicarbonate. The reaction was stopped by adding 250 µL of 10% (serum) or 4.6% (skin mucus) trichloroacetic acid (TCA). The mixtures were centrifuged (6000 g, 5 min) and 100 µL of the supernatants transferred to a flat-bottomed 96-well plate. Finally, 100 µL of NaOH 1 N (serum) or 0.5 N (skin mucus) were added. Optical density was read at 450 nm using a plate reader. Serum or skin mucus were replaced by trypsin (5 mg mL-1, Sigma-Aldrich) for the positive controls (100% of protease activity) or by ammonium bicarbonate buffer for the negative controls (0% of protease activity). Activity for each sample was expressed as % protease activity in relation to the controls.
2.14.5. Antiprotease activity
Antiprotease activity of serum was determined by the ability of serum to inhibit trypsin activity [39]. Briefly, 10 µL of serum samples were incubated (10 min, 22ºC) with the same volume of standard trypsin solution (5 mg mL-1) in 100 mM ammonium bicarbonate. Afterwards, 100 µL of 100 mM ammonium bicarbonbate buffer and 125 µL of buffer containing 2% (serum) or 0.7% (skin mucus) azocasein, samples were incubated (2 h, 25ºC) and, following the addition of 250 µL of 10% (serum) or 4.6% (skin mucus) TCA, a new incubation (30 min, 25ºC) was done. The mixtures were centrifuged (6000 g, 5 min) and 100 µL of the supernatants transferred to a flat-bottomed 96-well plate. Finally, 100 µL of NaOH 1 N (serum) or 0.5 N (skin mucus) were added. Optical density read at 450 nm using a plate reader. For the positive control (0% of protease activity and 100% antiprotease activity), ammonium bicarbonate buffer replaced serum and trypsin, and for a negative control (100% of protease activity and 0% of antiprotease activity), ammonium bicarbonate buffer replaced the serum or skin mucus samples.
2.15. Statistical analyses
The results are expressed as means ± standard error of mean (SEM). The normality of the variables was confirmed by the Shapiro–Wilk test and homogeneity of variance by the Levene test. For in vitro studies, statistical differences among the four groups of treatments were assessed by one-way ANOVA analyses, followed by the Tukey or Games-Howell test, depending on the homogeneity of the variables. For its part, in the in vivo studies data were statistically analysed by Student’s t test to determine differences between experimental groups at each sampling point. Non-normally distributed data were log-transformed prior to analysis and a non-parametric Kruskal-Wallis test was used when data did not meet parametric assumptions. The significance level was 95% in all cases (p < 0.05). Statistical analyses were conducted using SPSS 19.0 and differences were considered statistically significant when p < 0.05. All measurements were performed in three replicates.
3.
Results
3.1. Effect of purslane extracts on HK leucocytes
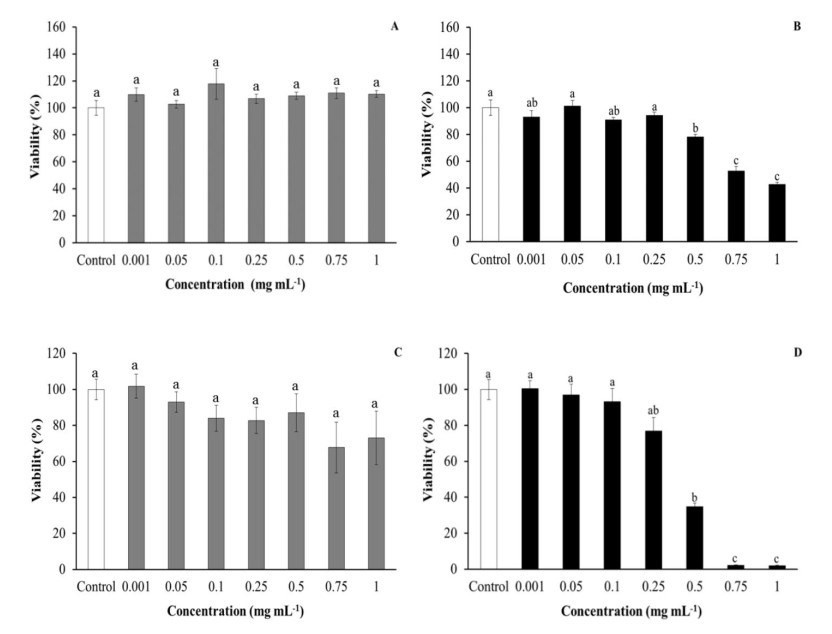
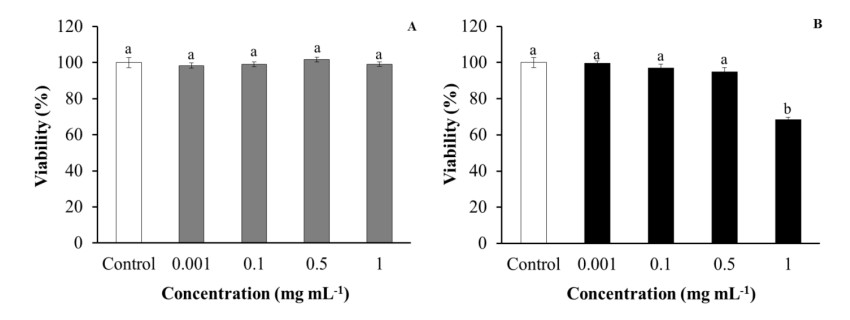
Leucocyte viability was evaluated in order to discard the cytotoxic effects of purslane extracts that could affect the cells. Regarding aqueous extracts, no effects were observed on HK leucocyte viability after being incubated with purslane extracts, with respect to control leucocytes. However, ethanolic extracts produced statistically significant decreases in HK leucocyte viability when they were incubated with the highest tested concentration (1 mg mL-1) in comparison with the control group (Figure 1).
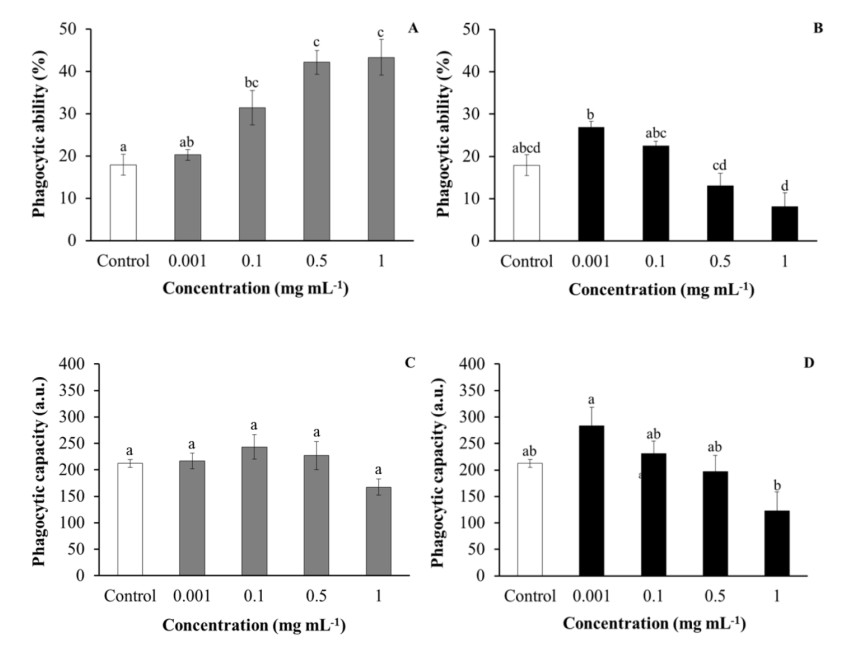
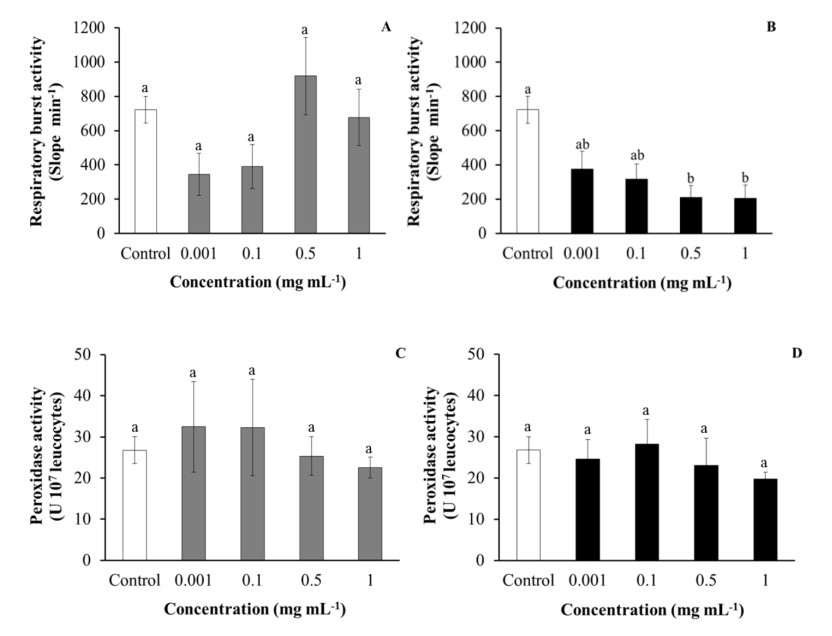
Regarding the phagocytic ability of HK leucocytes, after being incubated with purslane, aqueous extracts were significantly increased in a dose-dependent manner with respect to the values obtained for the control leucocytes. However, the incubation of HK leucocytes with low concentrations (0.001–0.1 mg mL-1) or medium to high concentrations (0.5–1 mg mL-1) of ethanolic extracts increased and decreased, respectively, the phagocytic ability of the leucocytes, although not always in a significant manner in comparison with the control leucocytes (Figure 2). No significant effects were observed on the HK leucocytes’ phagocytic capacity after incubation with either aqueous or ethanolic purslane extracts with respect to control leucocytes (Figure 2). Similarly, no significant effects were observed in HK leucocytes respiratory bursts and peroxidase activities after being incubated with purslane aqueous extracts with respect to control leucocytes, while incubation with ethanolic extracts significantly decreased respiratory burst activity with respect to the control leucocytes (Figure 3).
3.2. Cytotoxicity of purslane extracts on fish cell lines
No significant differences in either SAF-1 or PLHC-1 cell viability were observed after incubation with purslane aqueous extracts. However, medium–high concentrations (0.5–1 mg mL-1) of ethanolic extracts resulted as cytotoxic for SAF-1 cell and PLHC-1 cell viability, in a statistically significant way, with respect to the cell viability of control cells (Figure 4).
3.3. Bactericidal activity of purslane extracts
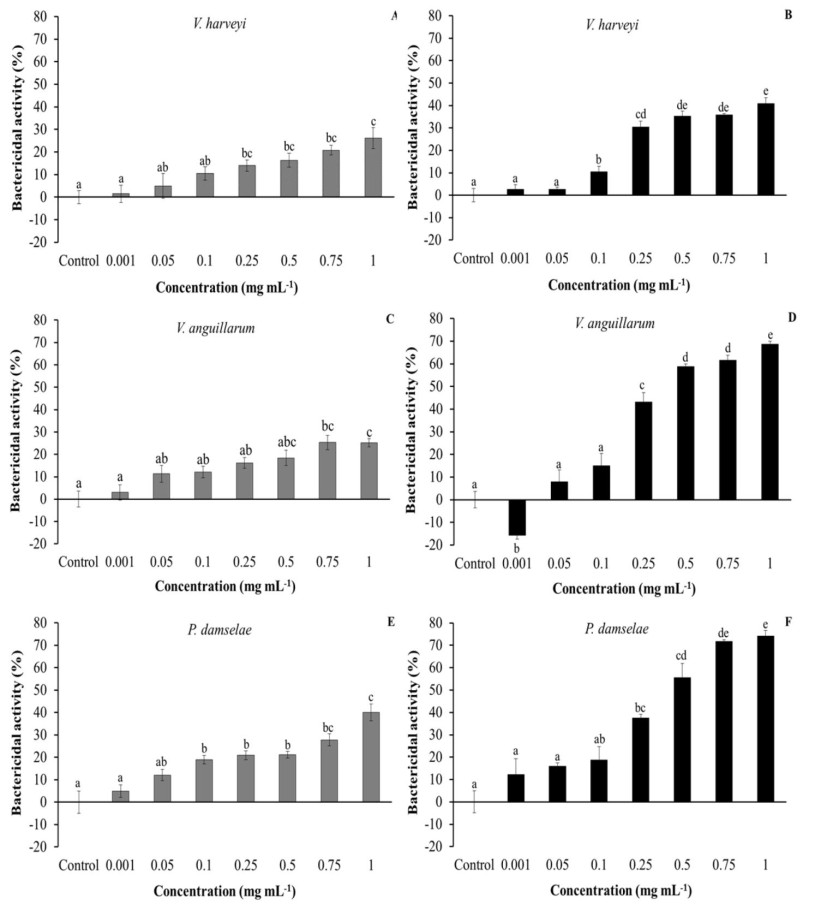
Both aqueous and especially ethanolic extracts of purslane had bactericidal activity against the three tested bacteria (Figure 5). Regarding V. harveyi, concentrations from 0.25 mg mL-1 of aqueous extracts and 0.1 mg mL-1 of ethanolic extracts showed bactericidal activity in a significant manner (Figure 5A, 5B). In the case of V. anguillarum, while only high concentrations (0.75–1 mg mL-1) of aqueous extracts showed significant activity, low concentrations starting from 0.25 mg mL-1 of ethanolic extracts showed very strong activity. Curiously, the lowest concentration (0.001 mg mL-1) of ethanolic extracts significantly increased bacteria viability (Figure 5C, 5D). Finally, in the case of P. damselae, concentrations from 0.1 mg mL-1 of aqueous and 0.25 mg mL-1 of ethanolic extracts showed bactericidal activity in a significant manner (Figure 5E, 5F).
3.4. TAA of purslane extracts
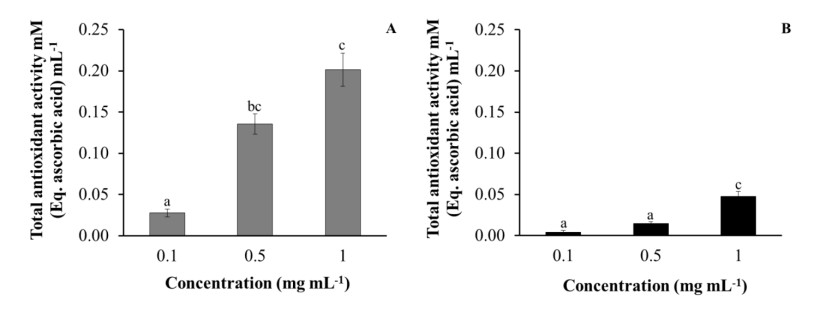
Both aqueous and ethanolic purslane extracts had antioxidant activities in a dose-dependent manner determined by a TAA assay, this property being particularly high in aqueous extracts (Figure 6).
3.5. Effect of supplemented diet on growth performance
Experimental diets were well-accepted by gilthead seabream and no mortality was recorded during the trial. Seabream body weight increased from an initial value of 49.6 g and 47.7 g to 72.1 g and 67.2 g for the fish-fed control and the supplemented diet, respectively (Table 1). No significant variations were detected in WG, SGR and CF of the purslane-fed fish with respect to the results obtained for the control fish at any assayed experimental time, although the values of WG and SGR were slightly higher in the control diet fish at the end of the trial (30 days).
3.6. Effect of supplemented diet on cellular immunity
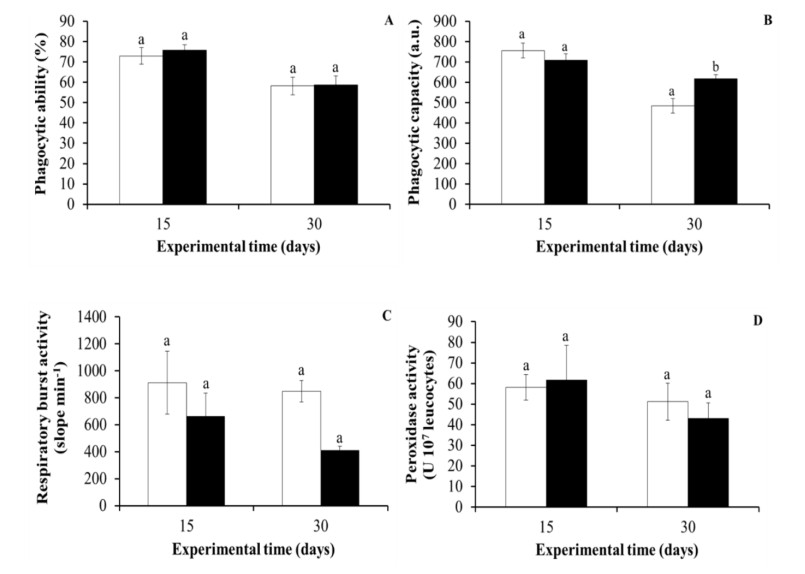
Regarding the results for cellular immunity, the phagocytic ability of HK leucocytes did not vary significantly in the fish with the supplemented diet compared to the control fish (fed with a non-supplemented diet) at any experimental time. However, the phagocytic capacity of HK leucocytes was significantly higher in the purslane-fed fish for 30 days than in the control group. Similar to phagocytic ability, the peroxidase and respiratory burst activities of the HK leucocytes were not significantly affected by the dietary administration of purslane to gilthead seabream specimens for 15 or 30 days (Figure 7).
3.7. Effect of supplemented diet on humoral immunity
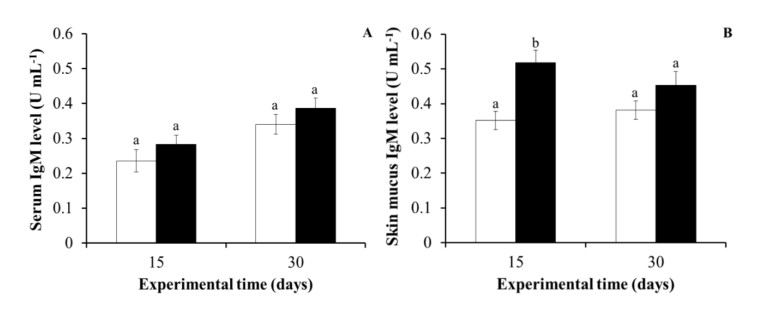
In the case of humoral immunity, while no significant differences were observed on the total serum IgM levels in the purslane-fed fish for 15 or 30 days in comparison with the values obtained in the control fish, the skin mucus IgM levels of the purslane-fed fish for 15 days were significantly higher than those levels detected in the skin mucus of the control fish at 15 days. Nevertheless, significant increments were not observed in the purslane-fed fish at 30 days (Figure 8).
No significant differences were observed in the serum and skin mucus lysozyme, peroxidase, protease and antiprotease activities of the purslane-fed fish, in comparison with the values obtained for the same activities in the control fish for any assay (Tables 2 and 3).
4.
Discussion
Medicinal plants, which have been used in the traditional medicine of many cultures for thousands of years [40], have become a potent and promising alternative to the use of antibiotics to prevent and/or control fish diseases and infections [2,3,4,5,6,41]. Purslane was selected for the present study due to its medicinal properties and abundance in Spain as well as the absence of any use of this plant considered, at present, only as bad weed. Our results could contribute to revalorising this plant, which is very abundant in Mediterranean countries, and help to consider it as a food additive for farmed fish or other farmed animals. It is known that the chemical compounds extracted from a plant depend on the concentration and polarity of the solvents used [42]. For this reason, we used water and ethanol, two solvents with different polarities. In this sense, alcoholic or organic solvents are better than water solvents to obtain high phytochemical concentrations [3].
Regarding the in vitro study, only the most concentrated ethanolic extract used (1 mg mL-1) significantly decreased gilthead seabream HK leucocytes’ viability, resulting in toxicity for the cells. However, the present results demonstrated that the leucocytes incubated with aqueous extracts increased their phagocytic ability in a dose-dependent manner, while no significant effects were detected on their phagocytic capacity. Furthermore, incubation with ethanolic extracts did not affect phagocytic ability or capacity on such cells. These results suggest that components present in the aqueous extracts can activate the phagocytosis of HK leucocytes, which is considered one of the main cellular activities involved in innate immunity against bacteria. The observed stimulation of phagocytosis could be due to several processes, such as the stimulation of the expression of cellular receptors that recognise pathogen-associated molecular patterns, or the production of cytokines, antibodies or the possible increase of pseudopod emissions. Therefore, aqueous extracts of purslane used at a suitable concentration enhance cellular immunity by increasing phagocytic activity. In order to explain these results, previous studies have established that vitamins C and B-complex, alpha-linolenic acid [43], tannins and alkaloids [44] could present immunostimulatory effects by increasing the human T helper 1–T regulators/T helper 2 lymphocytes ratio (Th1–Treg/Th2) [44].
The results of respiratory burst activity showed that while purslane aqueous extracts did not have any significant effect on this activity, decreasing effects were observed after being incubated with high concentrations of ethanolic extracts (0.5 or 1 mg mL-1). Besides, no significant variations were detected in the peroxidase activity content of leucocytes after being incubated with purslane extracts. Results concerning both activities can be explained by taking into account the presence of potent antioxidant compounds in both purslane extracts, which could eliminate the reactive oxygen species produced as a consequence of the leucocyte activation. This might indicate that purslane extracts could activate the phagocytosis activity of immune system cells and, at the same time, help to eliminate the reactive oxygen species that can cause damage and oxidative stress to fish. According to the purslane in vitro effects on leucocytes, the aqueous extracts had better immunostimulant properties than the ethanolic extracts.
To the best of our knowledge, this is the first study analysing the in vitro effects of purslane extracts on fish cell lines. The incubation of SAF-1 cells with purslane extracts demonstrated that aqueous extracts are not toxic or innocuous for fish cells, in agreement with the results obtained testing purslane aqueous extracts on rat embryo fibroblast cell viability [45]. Furthermore, the present results also demonstrate that low concentrations of ethanolic extracts from aerial parts of purslane did not affect the viability of human erythrocytes [46]. New biochemical studies are needed in order to identify which compound(s) present in purslane ethanolic extracts could be responsible for the toxicity caused to immune cells.
As regards PLHC-1 cells, incubation with a medium–high concentration of ethanolic extracts led to a significant decrease in cell viability. The cytotoxic activity of purslane extracts and oils was previously demonstrated against murine mammary adenocarcinoma [45] and human colorectal, breast and hepatocellular and lung adenocarcinoma cells [47,48,49,50], while water-soluble polysaccharides and their sulphated derivates inhibited mouse and human cervical carcinoma proliferation [51,52]. In summary, ethanolic extracts had greater cytotoxic activity against tumour cells than aqueous extracts, probably because they were able to inhibit tumour cell proliferation through cell cycle arrest, DNA damage and apoptosis [48,51,52]. Although the strong bactericidal activity of purslane extracts against Gram-negative and Gram-positive bacteria is known [11,12,13,16,46,53,54], there are no previous studies about fish pathogens and the present study corroborates this important property of purslane extracts, also in marine fish pathogenic bacteria.
To conclude with the in vitro results, in agreement with previous studies [45,53,55], both aqueous and ethanolic purslane extracts showed noticeable dose-dependent antioxidant activity, which was particularly evident in the aqueous extracts. This powerful antioxidant activity of purslane extracts has already been established in previous studies, where aqueous and ethanolic purslane extracts from aerial parts had a cytoprotective effect against oxidative stress in RBCs [56]. In addition, aqueous extracts showed a cytoprotective effect on human lymphocytes incubated with H2O2 by decreasing DNA damage and increasing cell proliferation, while 80% ethanolic extract had no effect [57]. The same results were obtained using purslane polysaccharides, which decreased RBC hemolysis induced by H2O2 in a dose-dependent manner and rat spleen T and B lymphocyte damage [58]. In addition, we analysed the dietary administration of purslane to gilthead seabream growth performance and immune status.
In general, medicinal plants can improve the digestibility and availability of nutrients, increasing food utilisation and growth rates and fish nutritional status is considered one of the most important factors to determine fish health status [59,60,61]. (Zhang et al., 2002; Karimi et al., 2004; Chan et al., 2000; Rashed et al., 2003)In the present study, the purslane concentration chosen was based on a pilot study where three inclusion levels were tested (1, 2 and 3%), the best results being obtained in fish fed at 2% for 15 days (data not published). Recently, it has been demonstrated that purslane has a high nutritional quality. Indeed, it possesses higher beta-carotene, ascorbic acid and alpha-linolenic acid levels than any other cultivated vegetable and it has one of the highest omega-3 fatty acid levels among plants [62]. The results of the present work showed that 2% of purslane included in the gilthead seabream diet for 30 days does not compromise fish growth performance. Considering that purslane contains phytate and oxalate, which are considered antinutritional factors [63], new studies are needed to evaluate the feed efficiency and digestive enzyme activities in the fish liver or gut among purslane-fed fish.
Among the beneficial effects of plants are their immunostimulant properties. For this reason, we studied the effect of a purslane diet on gilthead seabream immunity levels. At the end of the trial, the phagocytic capacity of the HK leucocytes of purslane-fed fish was higher than that of fish fed with the control diet. These results indicate that, while the number of active phagocytes in HK was similar among both sets of fish, the phagocytes from HK of the purslane-fed fish were more avid, which might improve the fish’s defence against infection, a hypothesis that needs to be addressed in future studies, which corroborates the present in vitro results and is in agreement with the previous results of our team after the dietary administration of dehydrated lemon peel (1.5–3%) or fenugreek (10%) to gilthead seabream diets [7,8]. Perhaps this beneficial effect is mediated by cytokines such as the macrophage activating factor secreted by peritoneal lymphocytes [64,65]. These positive effects of different plants on phagocytosis, a very important process of cellular immunity, underline the suitability of using such additives in farmed fish diets. However, as regards leucocyte peroxidase and respiratory burst activities, no significant effects were observed in purslane-fed fish with respect to the control fish, which could be also related to the potent antioxidant properties present in purslane extracts, as was previously discussed.
In our study, several immune molecules and immune-related enzymes were evaluated in serum and skin mucus of gilthead seabream. Skin is the outermost organ of the body and the first line of defence against external pathogens. It constitutes a crucial immune barrier and has an external layer of mucus that presents numerous immune factors [66,67]. Most of the studied humoral immune activities were not significantly affected by purslane administration. Antibodies represent the major component of the humoral immune system and they are known to play an adaptive role in neutralising and destroying invading pathogens [68]. IgM is the most common immunoglobulin present in the serum and mucus of fish, and it plays a pivotal role in systemic immune response [69]. In the present study, fish fed with a purslane-supplemented diet for 15 days showed higher IgM levels in skin mucus than the control group, whereas the levels remained unchanged in the serum. Our results agree with previous studies, where gilthead seabream fed with other plant-based dietary supplements also increased the skin mucus IgM levels [61,70]. On the other hand, it should also be taken into account that several datasets support the idea that immunoglobulin T (IgT) is the most important immunoglobin on the mucosal surfaces in fish. For instance, Cerezuela et al. reported an up-regulation of IgT gene expression in the skin of fish fed with a diet supplemented with date palm fruit extract for 30 days, whereas the expression of the igm gene was unaffected [70]. Further studies are necessary to understand the mechanisms involved in this respect.
Lysozyme and peroxidase were also studied due to their implications in bactericidal activity. Lysozyme acts on cell wall peptidoglycan, causing bacteriolysis and preventing bacterial growth [71] and activating the complement system and phagocytes by acting as an opsonin, as well as to display anti-viral and anti-inflammatory properties [72], while peroxidase activity uses the antioxidant power of the hydrogen peroxide generated in other reactions to produce hypochlorite, which leads to the production of chloramines, which are oxidative substances that attack microorganism membranes [66]. No significant differences were obtained for lysozyme and peroxidase activity, neither in the serum nor in the skin mucus of fish fed with the supplemented diet compared with the values obtained for the control fish at any assayed time. The results seem to demonstrate that the levels of these enzymes are not modulated by the inclusion of purslane into the diet.
Similarly, protease and antiprotease activities in serum and skin mucus did not show significant changes between the fish fed with different diets. The role of proteases and antiproteases has been related to the defence against bacterial or parasite infections [73] due to their enhancing the production of other immunological components such as immunoglobulins and antimicrobial peptides [74,75]. Proteases perform this task by directly degrading pathogens, while antiproteases are blood proteins that act against pathogen proteolytic proteins [73]. Contrarily to our results, several studies have described changes in proteases and antiproteases in serum and skin mucus following plant-based immunostimulation, pointing to their importance in systemic and mucosal immunity [8,70,76,77,78]. More studies are needed to identify the main plant bioactive substances that could alter the protease and antiprotease levels in fish.
Finally, purslane inclusion in a gilthead seabream diet did not affect either seric or skin mucus levels of lysozyme, proteases and antioproteases (all of which are known to have anti-inflammatory properties). These results seem to support that 2% of purslane in the diet had no effect on the gilthead seabream inflammatory response. Similarly, few inflammatory signals (such as the increase of plasma NO levels and lysozyme activity) were detected in European seabass (Dicentrarchus labrax) after the administration of different plant proteins [79].
5.
Conclusions
This work is the first study in which the effect of purslane in gilthead seabream has been analysed. Purslane extracts present many interesting biological activities, including immunostimulant, bactericidal and antioxidant properties. Furthermore, the addition of 2% purslane powder into the gilthead seabream diet for 30 days had no negative effects on fish growth performance and improved fish immunity. These findings make purslane a possible plant to be used as additive to farmed fish instead of other chemical compounds.
Author contributions
García-Beltrán JM carried out the preparation of purslane extracts and the in vitro tests carried out in this work, while Cámara-Ruiz M and Guardiola FA were in charge of the in vivo study. García-Beltrán JM and Cámara-Ruiz M carried out the bibliographic search and wrote the article draft while Esteban MA was in charge of supervising and review all the work.
Acknowledgements
The authors are grateful to Ms. A.I. Salvá for the technical assistance provided during the experiment. This work was supported by the Spanish Ministry of Economy and Competitiveness (Grant no. AGL2014-51839-C5-1-R) co-funded with Fondos Europeos de Desarrollo Regional / European Regional Development Funds) and by the Fundación Seneca de la Región de Murcia (Grupo de Excelencia grant no. 19883/GERM/15).
Conflict of interest
The authors declare no conflict of interest.









 DownLoad:
DownLoad: