1.
Introduction
Crops are exposed to many unfavorable abiotic stresses that reduce their productivity. Soil salinization is one of the most common abiotic stresses that inhibits the growth and yield of cultivated plants [1]. High soil salinity negatively affects the quality and quantity of a crop production: it reduces the number and area of leaves, wet and dry biomass, length of shoots and roots, and also inhibits seed germination, growth rate, chlorophyll content, photosynthesis, transpiration rate, water and ion metabolism in cells, osmotic pressure and complicates the qualitative and quantitative assessment of these parameters [2,3]. In a saline environment, the number of primary and secondary shoots are significantly reduced, as well as the number of spike shoots. Furthermore, salinization promotes wheat lodging [4]. In order to grow and develop on saline soils, a plant needs to regulate the accumulation of salt ions at the cellular level [5]; this can be done in several ways: resistance to osmotic stress, limited accumulation of Na+ cations and Cl- anions, and resistance to the accumulation of these ions [6]. Plants respond to salinity in two phases: ionic, which is slow, and which accelerates the aging of mature leaves; and osmotic, which is fast and inhibits the growth of young leaves.
High salinity negatively affects the metabolism of plants, mainly due to violation of the ionic and osmotic equilibrium of cells. In saline soils, a high level of Na+ ions inhibits growth and can lead to plant death [7]. Salinity causes a change in the K+:Na+ ratio. Exogenous Na+ can negatively affect the influx of K+ into cells, which is necessary for plant growth. Changes in the concentration of K+ ions can disturb osmotic balance, stomatal function, and the function of certain enzymes. Salinity leads to an increase in the concentration of Nа+ and Cl- in the cytosol, which can ultimately harm the cell. High concentrations of Na+ ions (above 100 mM) are toxic to cellular metabolism and can inhibit the activity of many important enzymes, cell division and multiplication, membrane disorganization, and osmotic balance, which ultimately can lead to an inhibition of growth. High concentrations of Na+ ions can also lead to a decrease in photosynthesis and the formation of reactive oxygen species (ROS). High concentrations of Na+ ions have a toxic effect inside plants. Salt can be concentrated in old leaves, causing the leaves to die and this is crucial for the survival of the plant as a whole [6]. It should be emphasized that plants can respond to salt stress as a whole plant, as organs, or as individual cells.
Wheat (Triticum aestivum Host.) is, by volume of grain production and the sown area, the most commonly cultivated grain crop in the world [1]. For regions with primary and secondary salinization, the determination of wheat salt tolerance potential is one of the most important in plant breeding. It is known that the more salt tolerant varieties of wheat can accumulate Na+ and Cl- to a limited extent in leaves and roots, and become adjusted to the concentration of salts in the environment. At the same time, salt ions must only affect metabolism in a limited way, without disturbing it. If these ions do not accumulate in vacuoles, and a low concentration is maintained in the cytoplasm, wheat tissues remain resistant to salinization. In order for wheat varieties to be tolerant to salinization, cells must maintain an order of magnitude lower concentration of Na+ ions in root cells and a higher concentration of K+ ions in vacuoles as compared with sensitive varieties [8,9]. Within the genus, various types of wheat tolerate saline conditions to varying degrees, and halophytes are found among them. By crossing salt-tolerant species with non-salt-tolerant species, new salt-resistant wheat varieties can be obtained. The salinity marker in this case was the accumulation of Na+ and Cl- ions. As part of the above mentioned study, various wheat genomes were studied: tetraploid and hexaploid, durum wheat, soft wheat, and their wild relatives (for example, aegilops and elongated wheatgrass). Halophytes accumulate Na+ cations and, in some cases, Cl- anions to a limited extent, and their ancestors were used to produce synthetic hexaploids. It is noteworthy that in the bluegrass family, several wild species can be crossed with hard and soft wheat. Relatives of halophytes, such as high wheatgrass, were used to obtain amphiploids, lines of addition and substitution of disomic chromosomes, as well as recombinant lines in wheat [10]. Even a low degree of salinity reduces wheat productivity, through a reduction in grains per spike and especially the mass of grains [11]. The toxicity of chlorine is stronger than sulfate, and chloride salinization at low levels of up to 5–7 atm reduces wheat quality and yield. With an increase in the degree of salinization, osmotic pressure increases, which leads to a leveling of differences between types of salinization [12]. Under the influence of two stress factors-osmotic and toxic (including oxidative)-significant damage to plant cells takes place [13]. The consequences of oxidative stress, which is the generation of reactive oxygen species (ROS) [14,15] lead to programmed cell death, because cell membranes are damaged, along with protein degradation, enzyme inactivation, base modifications, and DNA breaks [16,17,18,19,20,21]. The ability of cells under oxidative stress to stop cell division is also an important response to stress. In the presence of sodium chloride (0.2 M), the cell cycle in sorghum roots was observed to lengthen [22]. Plant cells under the influence of oxidative stress show a temporary halt in the cell cycle. Thus, the study and improvement of diagnostic methods in the early stages of plant development seem to be quite relevant.
The aim of this study was to identify morphological and cytological markers of wheat resistance when exposed to high concentrations of NaCl (sodium chloride) and Na2SO4 (sodium sulfate), using differential diagnosis of tissue damage at different stages of seedling growth.
2.
Materials and methods
2.1. Plant materials
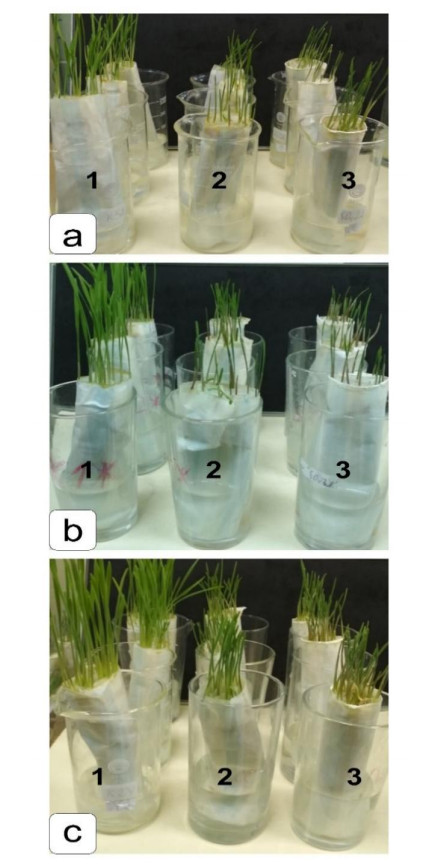
Three varieties of soft wheat (Triticum aestivum Host.) were studied, including the spring forms of Agata and Uchitel’, and the winter form Zhemchuzhina Povolzh’ya, . The seeds were obtained from the Timiryazev Agricultural Academy collection [23]. The sensitivity of plants to NaCl and Na2SO4 salinity was assessed on seedlings grown in a roll culture. Paper rolls were placed in glasses, 150 mL of each solution was added to each roll: water (control), 100 mM NaCl solution or 80 mM Na2SO4 solution, which corresponded to an osmotic pressure of 4 atm. Glasses were placed in a climatic chamber. Cultivation was carried out at 24 ℃ with artificial lighting 5000 LK, humidity 60%, photoperiod 10/14 day/night. Biometric measurements were performed after 6 days.
2.2. Staining using trypan blue
Intravital staining of coleoptiles with 0.5% aqueous solution of trypan blue (AppliChem GmbH, Germany) was carried out for 5 min. Photography was captured using a Color Vien digital camera (Germany) on an Olympus BX51 microscope, with a ×10 lens.
2.3. Fluorescence microscopy
The root tip (4–5 mm) of the wheat germ (5 pcs) was separated and placed on a glass slide in a drop of water. An aqueous solution of 5-(and-6)-carboxy-2’, 7’- difluorodihydrofluorescein diacetate (carboxy-H2DFFDA) (Thermo Fisher Scientific, Waltham, MA, USA) was used at a concentration of 25–50 nM; the incubation time was 30 min for intravital imaging of ROS in cells.
The preparations were analyzed using an Olympus BX51 microscope (Japan) with a ×10 objective, at a wavelength of 490 nm, and a Color Vien digital camera (Germany).
2.4. Cytophotometric analysis
The root tips were fixed at a size of about 4–5 mm for 3 h in a mixture of ethanol and acetic acid (in a ratio of 3:1). The roots were macerated for 2 h at 37 ℃ in a solution containing 0.4% cellulase (Sigma, USA) and 0.4% pectinase (Merc, Germany). Hydrolysis was carried out in 5N HCl (40 min at 22 ℃), then for 2 h the cell suspension was stained with Schiff’s reagent (Merck, Germany). Nuclear DNA content was measured on an SMP-20 cytophotometer (Opton) with a probe of 0.16 mm, a ×16 lens and a ×10 eyepiece. For the control, nuclei of fissile cells at metaphase (4C) or telophase (2C) were used. For each experimental point, the sample included at least 300 root cell nuclei.
2.5. DNA isolation
Six-day coleoptiles from different wheat varieties and grown under different conditions were thoroughly ground in a mortar with liquid nitrogen to the resulting fine powder, then lysing buffer (50 mM Tris-HCl, pH 7.5; 25 mM Ethylene diamine tetraacetic acid (EDTA) disodium salt, 1% Sodium dodecyl sylphate (DS-Na) was added, suspension was thoroughly mixed and the mixture was incubated for 30 min at room temperature. Subsequently, NaCl was added to the mixture to a concentration of 1 M, and it was deproteinized by shaking it gently with a mixture of chloroform-isoamyl alcohol (10:1, v/v). The obtained DNA samples were treated with ribonuclease A (50 μg/mL for 20 min at 37 ℃ and proteinase K (10 μg) for 1 h at 37 ℃. DNA was further treated by adding three volumes of 96% ethanol [24].
2.6. Electrophoresis in agarose
DNA preparations were analyzed by horizontal electrophoresis. The isolated and purified DNA preparations (2 μg each) were dissolved in Tris-borate buffer (10 mM, pH 8.0) containing 10 mm EDTA and subjected to horizontal electrophoresis for 2 h in a 1.2% agarose gel with an electric tension of 2–3 V/cm in 0.09 M Tris-borate buffer, pH 8.3, containing 0.5 μg/mL ethidium bromide. After electrophoresis, the products of DNA hydrolysis were visualized under UV light.
2.7. Statistical Analysis
To evaluate the statistical parameters, standard methods were used within the programs Statistica 6.0, STATAN and standard Microsoft Excel software. The mean values (n = 50) and their standard deviation are shown according to Student’s criterion, p < 0.05.
3.
Results and discussion
3.1. The changes morphometric parameters of different varieties wheat in different growing conditions
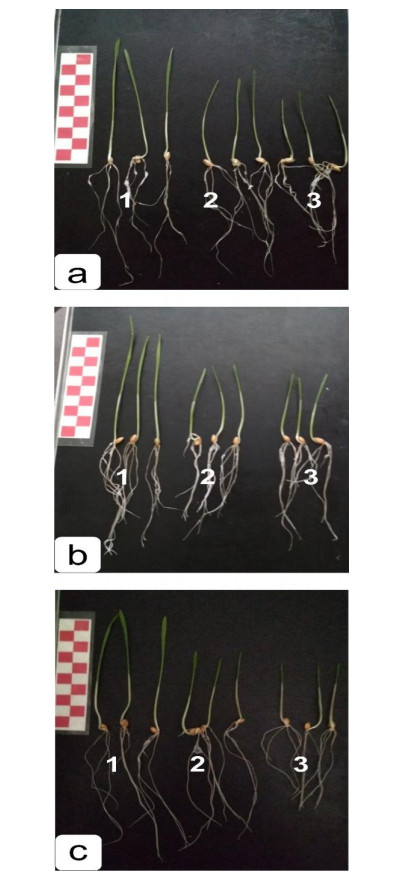
One of the visible symptoms of exposure to NaCl and Na2SO4 is damage to seedling growth (Table 1). Despite the use of different salts, an inhibition of root and shoot growth was observed, by the strength of the manifestations of which one can be concluded about the toxicity of the salt [23].
During early developmental stages, the biomass under the influence of chloride salinization was inhibited in all studied varieties, decreasing by 2%–24% compared with the control. Meanwhile, under the influence of sulfate salinization the speed decrease was by 11%–29% (Table 1). Naturally, a decrease in the development of biomass cannot but affect the development of roots and seedlings. Results showed that the length of the main root decreased by 12%–20% under the influence of chloride salinity, and by 12%–24% under the action of sulfate salinity. The height of the seedlings under the influence of chloride salinity decreased by 30%–39%, and with sulfate by 35%–41% (Table 1). This shows, firstly, that a decrease in the biomass accumulation of seedlings in the presence of chloride and sulfate salinization occurred, to a greater extent, in the aerial part of the seedling (Table 1). In this regard, it was most likely that chloride and sulfate salinization inhibited the overall metabolism of shoot tissues.
Chloride salinization had a less pronounced effect on the growth of biomass in the variant Agata on Day 6 of development; it differed from the control value by 2%, while sulfate varied by 11%. (Table 1, Figures 1 and 2). In addition, an analysis of the shoot lengths of control and experimental plants showed that the NaCl and Na2SO4 salts caused a slight decrease in root length (by 12%). Ambiguous data were obtained for dry biomass. For Agata, this indicator decreased only with chloride salinity, whereas dry biomass increased in Uchitel’ and Zhemchuzhina Povolzh’ya cultivars under chloride and sulfate salinization, which is typical of stress exposure. Thus, the inhibitory effect of chloride and sulfate salinization is to reduce root and shoot growth compared with the control and, as a result, to decrease biomass.
According to biometric indicators, under the influence of NaCl and Na2SO4, the studied wheat varieties can be divided into three groups: A resistant variety (Agata), a medium-resistant variety (Uchitel’) and a highly sensitivity variety (Zhemchuzhina Povolzh’ya).
According to these results, we drew the following conclusions. Since growing wheat in a saline environment leads to the inhibition of wheat root and shoot growth, it is logical that shorter shoots and roots would be obtained in variants grown within a saline media, over control variants. Comparing morphometric parameters, we concluded that Agata was the most salt tolerant wheat variety; its root growth was inhibited by 11%, and shoots by 30%. Growth inhibition in other varieties exceeded these latter indicators.
Wheat grown in a saline environment tends to gain a higher dry matter content than in a non-saline environment. Based on the obtained dry matter results, the most salt tolerant variety to chloride salinization was Agata; in a medium with NaCl, a decrease in the dry matter content was observed, which was not observed in other varieties. In turn, the same variety was also the most resistant to sulfate salinization; in the medium containing Na2SO4, the accumulation of dry matter was 15% higher than in the non-saline medium, which was lower than in the other varieties.
Since the inhibition of plant growth and the accumulation of dry mass in accordance with the resistance of wheat to a saline environment is obvious, this marker of wheat salt tolerance was quite accurate.
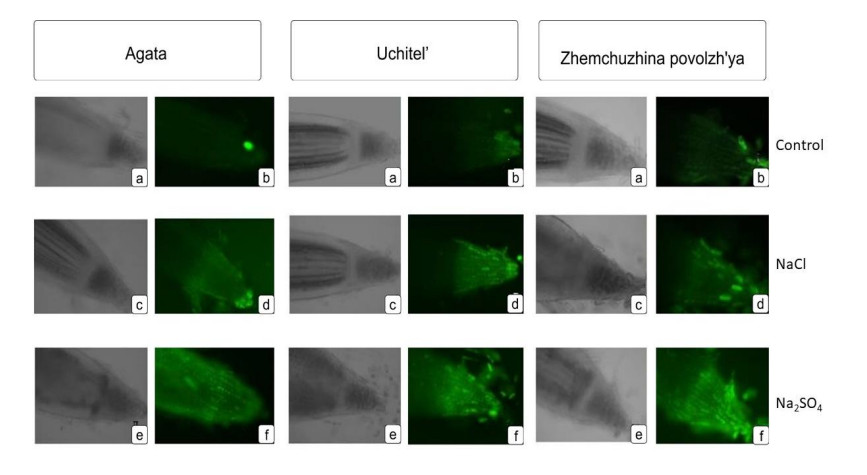
3.2. Influence of salinity on ROS release in plant cells
The ROS marker Carboxy-H2DFFDA was observed in roots after exposure to NaCl and Na2SO4, and more often with more intense fluorescence compared to the control. This indicated the activation of oxidative stress and increased ATF production in cells. An increased content of the fluorescent marker ROS Carboxy-H2DFFDA in root cells during salinization can initiate programmed cell death (PCD), as ROS homeostasis is disturbed in the cells [14,15,16].
Results reflecting the functional state of cells grown under the influence of various stress factors, using intravital markers in the future, could be effectively used for a rapid assessment of the oxidative status of plant root cells. Based on the totality of all the data, Agata was identified as the least sensitive to the negative effects of NaCl and Na2SO4, and was therefore potentially the most stable. The less stable and more sensitive varieties were Uchitel’ and Zhemchuzhina Povolzh’ya (Figure 3).
Since the release of ROS occurs in accordance with the level of oxidative stress in the cells, which corresponds to the stress resistance of wheat, this could be a reliable and accurate marker for determining and comparing the salinity resistance of different varieties of wheat.
3.3. Electrophoretic characterization of DNA of wheat varieties
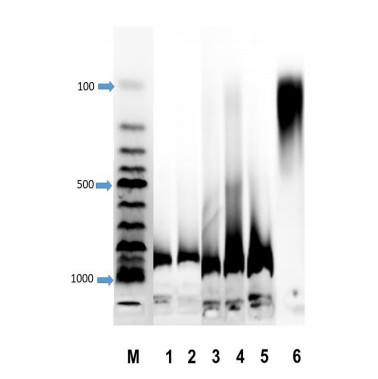
PCD is a natural process in the development of organisms. However, as a result of exposure to stress factors in plants, massive cell death can occur. One biochemical marker is the destruction of genomic DNA. The marker of dying cells in animals is internucleosomal DNA fragmentation with the formation of a “DNA ladder”. Wheat coleoptiles are an excellent model for studying the effects of abiotic stress on cell death. Although, owing to some features of cell structure and nuclear chromatin in plant cells, it is difficult to detect a clear classical picture of nuclear DNA fragmentation; the process of DNA degradation can also be detected in plant DNA to stressful effects. Figure 4 shows the electrophoresis data for agarose DNA from different varieties of wheat that were grown under different conditions (control, salt). Control samples of all varieties of wheat contain high molecular weight DNA. DNA from the variety Agata grown in the presence of 150 mM NaCl remained undegraded. In the Uchitel’ variety, there is intense degradation of DNA from wheat grown in the presence of NaCl. Finally, in the Zhemchuzhina Povolzh’ya variety, all the DNA of a coleoptile of wheat grown in the presence of salt is degraded to oligonucleotides (Figure 4). Thus, coleoptile DNA can serve as a good marker for characterizing the sensitivity of wheat to saline conditions. These data correlate with previously obtained data on the high sensitivity of coleoptiles to abiotic stresses [18].
3.4. Lifetime detection of dead cells
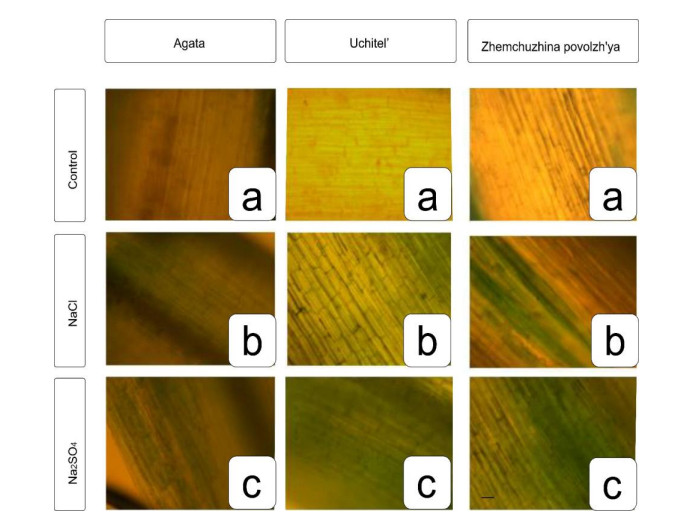
Staining of wheat samples with trypan blue allowed us to visualize the degree of damage to plant tissues (dead cells appeared darker in the photographs). In the control variant, cell death was minimal; in variants that had undergone salinization, it increased. When evaluating the photographs, it was noted that the most resistant variety to chloride and sulfate salinization was Agata wheat, (the number of dead cells was the lowest among all varieties), and Zhemchuzhina Povolzh’ya was sensitive to sulfate salinization, while Uchitel’ occupied an intermediate position (Figure 5).
3.5. Distribution of wheat root meristem cells by cell cycle phases
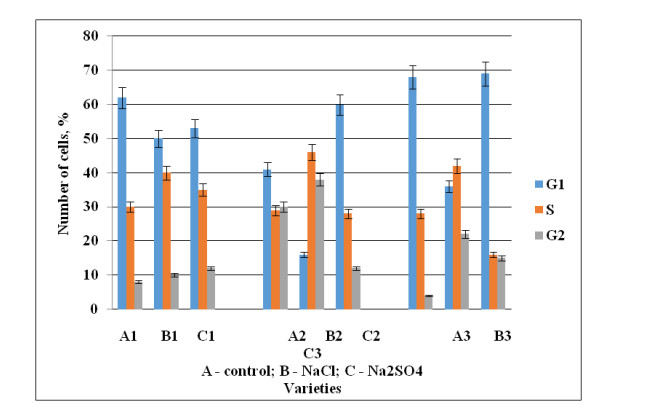
The effect of NaCl and Na2SO4 on the cell cycle in the cells of the root meristem of wheat seedlings was determined by cytophotometry. Seedlings of the three varieties exhibited differences in the passage of cell cycle phases. As we noted earlier, cells that are in the postsynthetic (G2) phase are more sensitive to stress and an increase in their number indicates stress exposure compared with cells in the presynthetic (G1) phase [22]. Salinity resistance was evaluated by the number of cells in the G2 phase. The accumulation of cells in one of the phases, leading to a cell cycle stop (block), has been observed in wheat seedlings of the Uchitel’ and Zhemchuzhina Povolzh’ya cultivars during sulfate salinization in the G1 phase, which inhibits division and stops the process of cells passing through the cycle (Figure 6). With sulfate salinization in the variety Agata, number of the cells in the G2 phase approaching 12%.
In the current experiment, for the varieties Zhemchuzhina Povolzh’ya and Uchitel’ in during chloride salinityи, the maximum number of cells were present in phase S (synthesis phase).
In the variety Zhemchuzhina Povolzh’ya, the number of cells in G2 during chloride salinity was higher compared to the control (5-fold), which indicated the lower resistance of the experimental plants and led to a decrease in growth and yield.
In the Agata cultivar, experimental plants at G2 differed to a lesser extent from the control plants, in comparison with plants of the Zhemchuzhina Povolzh’ya and Uchitel’ varieties, which determined their tolerance to salinization.
When analyzing wheat seedlings, the Agata variety was distinguished, under chloride salinity, by containing fewer cells in the G2 phase. Seedlings of the Zhemchuzhina Povolzh’ya and Uchitel’ varieties showed less stability in comparison with the Agata variety.
The results of cytophotometric analysis of the distribution of cells by the phases of the cell cycle showed that Agata wheat is tolerant to chloride and sulfate salinization better than seedlings of Zhemchuzhina Povolzh’ya and Uchitel’. These data can be used to optimize the selection of plants resistant to salinization in the early stages of their development.
4.
Conclusion
Wheat is one of the most important crops in the world. However, its safe production is seriously threatened by salinization, which lead to a significant decrease in annual yield and wheat quality. Accordingly, increasing the resistance of wheat to salt stress is one of the most important tasks of the breeding program. Comparison of wheat varieties using intravital markers in the future can be effectively used for rapid assessment tolerance of wheat to chloride and sulphate salinity. During the study, experimental data were obtained to identify stable genotypes that allowed us to characterize a systemic cellular response to stressful effects of an abiotic nature (using the action of edaphic stress as the toxic effect of NaCl and Na2SO4 in the roots of wheat seedlings.
New cytological criteria have been developed that determine, in the early stages of evelopment, the functional state of cells in whole plant roots, which will allow the manifestation and stress parameters to be assessed: stability/destruction of the structural organization of root cells and safety/destruction in coleoptile cells. Comparison of the functional state of cells using intravital markers of wheat genotypes of different resistance to chloride and sulfate salinization can subsequently be effectively used to evaluate the oxidative status of plant root cells.
Acknowledgments
Thank you Baranova E.N. for your technical assistance in writing this article. The reported study was supported by project RFBR 18–016–00150 and partially assignment 0574–2019–002 of the Ministry of Science and Higher Education of the Russian Federation.
Conflict of interest
All authors declare no conflicts of interest in this paper.









 DownLoad:
DownLoad: