1.
Introduction
On March 11, 2020, the World Health Organization (WHO) proclaimed the novel coronavirus outbreak, popularly known as SARS-CoV-2, a global pandemic [1]. As indicated by WHO (https://covid19.who.int/), the SARS-CoV-2 mortality rate is around 1.36%, with around 440,807,756 confirmed cases, including 5,978,096 deaths by March 2022 [2]. Studies have indicated that cancer patients have a greater vulnerability to SARS-CoV-2 infection caused by chemotherapy or immunotherapy [3]. In a large population sample study of more than 20,000 cancer patients, it was found that there was a significant increase in the SARS-CoV-2 infection among cancer patients [3]. According to recent studies, Individuals with cancer exhibited a greater likelihood of developing serious complications (such as intensive care unit hospitalization, invasive ventilation, or death) as opposed to those who did not have cancer (39 vs. 8%, p = 0·0003) [4]. With the advancement of medical science and tumor treatment methods in recent decades, the survival rate of cancer patients has continuously increased, and cancer is now considered a chronic disease rather than a terminal disease [5]. However, immunocompromised patients are at high risk as SARS-CoV-2 continues to mutate. Therefore, to better overcome these two diseases in the future, it is urgent to explore and clarify the internal molecular mechanism between them.
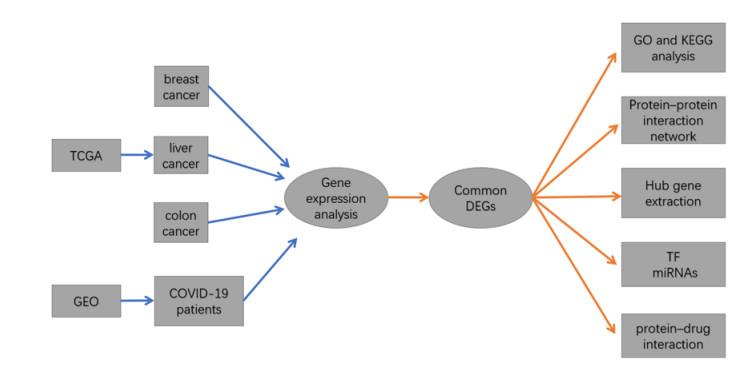
In this study, we selected breast cancer, liver cancer, and colon cancer, to explore the molecular mechanism between these three cancers and COVID-19. We chose breast cancer, liver cancer and colon cancer for analysis mainly for the following three reasons: 1) According to February 2022, the National Cancer Center of China announced the incidence of cancer in China in 2016 [6]. The current top five cancers are lung cancer, colon cancer, gastric cancer, liver cancer, and breast cancer. Therefore, there are a certain representative. 2) With the progress of surgical operations and the continuous development of comprehensive diagnosis and treatment of tumors, the overall prognosis of these three cancers (breast cancer liver cancer and colon cancer) is good at present. In our clinical work, we have found that many patients have been cured or have stable survival with tumor after undergoing surgery, radiotherapy and chemotherapy, immunotherapy and other treatments. However, gastric cancer patients have poor prognosis and low long-term survival rate. In our opinion, cancer will no longer be an incurable disease in the future, but will evolve into chronic diseases such as hypertension and diabetes. Therefore, we may have a greater chance of encountering these three types of cancer patients with COVID-19 in the long-term clinical practice. 3) The reason why we did not include lung cancer is that SARS-CoV-2 virus mainly affects lung, and the two diseases are in the same target organ. In our opinion, they need to be analyzed separately. We first identified the differentially expressed genes (DEGs) for the three distinct malignancies and the SARS-CoV-2 virus to discover the DEGs shared by all four disorders. This research is focused on the common DEGs, which serve as the core experimental genes. Additional investigations and analyses were carried out on the basis of these common DEGs and included a pathway analysis and an enrichment analysis to truly comprehend the biological mechanisms behind genome-based expression investigations. The extraction of hub genes from common DEGs was a critical step in the prediction of viable medicines. In addition, a network of protein-protein interactions (PPIs) was constructed using common DEGs to collect hub genes for further study. In this research, transcription modulators were identified predicated on the common DEGs. Last but not least, possible medications were proposed. The detailed procedure of our study is shown in Figure 1.
2.
Materials and methods
2.1. Datasets employed in this study
The transcriptome data of three common cancers of the breast, colon, and liver were acquired from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/). These include 1222 breast specimens (1109 cancer specimens, 113 normal tissue specimens), 546 colon specimens (502 cancer specimens, 44 normal tissue specimens), and 424 liver specimens (374 cancer specimens, 50 normal tissue specimens). The SARS-CoV-2 infection data were obtained from The Gene Expression Omnibus (GEO) database (ID: GSE147507) of the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/geo/) [7].
2.2. Determination of DEGs and common DEGs among COVID-19 and three different types of cancer
A statistically significant difference between distinct test settings at the transcriptional level was used as the criterion for determining genes that are differently expressed. Long-expression levels for DEGs were discovered utilizing the "limma" program with the Benjamini-Hochberg correction to adjust the false discovery rate. "DESEq2" of the R programming language (v 4.0.2) was also used to identify DEGs under a variety of testing settings. A p-value < 0.05 and |logFC| ≥1.0 was considered to be statistically significant. The common DEGs from four datasets were obtained by utilizing an online Venn analysis program known as Jvenn [8].
2.3. Analyses of gene ontology (GO) and pathway enrichment
A gene set enrichment analysis is a substantial analytical endeavor that aims to categorize basic biological knowledge, including cellular mechanisms of chromosomal sites related to several interconnected disorders [9]. Enrichr (https://maayanlab.cloud/Enrichr/) was utilized to conduct gene ontology, functional enrichment (cellular components (CC), biological processes (BP), and molecular functions (MF)), and pathway enrichment investigations. Enrichr is a web-based program for enriching gene sets [10] that is employed to study the biological processes and signaling paths underlying common DEGs. A total of four repositories were investigated for this research: the Kyoto Encyclopedia of Genes and Genomes (KEGG), BioCarta, Reactome and WikiPathways. These highlighted the genesis of the route categorization, which was used to identify the pathways shared by breast cancer, colon cancer, liver cancer, and COVID-19. Notably, the KEGG pathway is well recognized for its ability to comprehend metabolic processes while also demonstrating the significant value of genomic research. The standard criterion for measuring the top-listed pathways was a p-value < 0.05.
2.4. PPI network analysis and hub gene extraction
We then investigated the interactive relationships between proteins utilizing STRING (Search Tool for the Retrieval of Interacting Genes/Proteins), a web-based program that can be retrieved at https://string-db.org/. The use of STRING to study the PPI network of DEGs may aid in examining the associations across various genes. The STRING repository was utilized to create the PPI network of proteins generated from common DEGs in order to depict physical and functional relationships across three distinct cancer types and SARS-CoV-2. Hereafter, Cytoscape (v.3.7.1) was used to visualize and conduct subsequent investigations on the PPI network once it was loaded into the program. Cytoscape, a publicly accessible network visualization tool, is a versatile system in which multiple datasets are integrated to optimize for diverse interactions including PPIs, genetic connections, protein-DNA interplay, and so on. The PPI network is composed of edges, nodes, and interconnections between them. In this context, the nodes that are the most entangled are referred to be the hub genes. Cytohubba (https://apps.cytoscape.org/apps/cytohubba), a revolutionary Cytoscape plugin, is a tool for ranking and isolating central, potential, or targeted components of a biological network depending on a variety of network characteristics. Cytohubba is a collection of 11 techniques for evaluating networks from a variety of perspectives. Maximal Clique Centrality (MCC) is considered the most effective method [11]. The PPI network's top ten hub genes were discovered using the MCC approach, which was applied to the network. According to Cytohubba's near proximity ranking characteristics, the shortest accessible pathways linking hub genes were also identified.
2.5. Determination of transcriptional factors and miRNAs engaging with shared DEGs
Transcriptional factors (TFs) are proteins capable of binding to a specific gene and regulating the rate at which genetic information is transcribed. As a result, it is necessary to provide molecular understandings. The NetworkAnalyst (http://www.networkanalyst.ca) platform was utilized to discover topologically credible TFs from the JASPAR database (http://jaspar.genereg.net) that have a tendency to bind to common DEGs. NetworkAnalyst is a robust web-based resource for meta-analysis of gene expression profiles and the discovery of biological processes, functions, and interpretations of those findings [12]. JASPAR is a freely accessible repository of TFs from six taxonomic categories that may be used to identify TFs in numerous species [13]. MiRNAs that target gene interactions were also incorporated in the research to identify miRNAs that have the potential to bind to gene transcripts and so negatively impact protein production. MiRTarBase and TarBase are two of the most important repositories for evaluating the experimental validity of miRNA-target gene interplay [14]. MiRNAs from miRTarBase and TarBase that interplay with common DEGs were retrieved from the interactions between miRNAs and genes utilizing NetworkAnalyst, with a particular emphasis on topological analysis. In Cytoscape, interaction networks between transcription factors and genes and between microRNAs and genes were shown. This program assists researchers in filtering top miRNAs exhibiting elevated levels of expression and identifying biological roles and characteristics that may be used to develop a reliable biological hypothesis.
2.6. Assessment of applicant drugs
The most significant impact of this research is predicting protein-drug interaction (PDI) or identifying drug molecules. Utilizing Drug Signatures Database (DSigDB) via Enrichr, a drug molecule was discovered on the basis of the DEGs of SARS-CoV-2 and three distinct subtypes of cancer. DSigDB is a worldwide library for identifying targeted pharmacological compounds that are associated with DEGs [15]. Enrichr's "Diseases/Drugs" feature provides easy access to this database, which comprises 22,527 gene sets.
2.7. Data availability
The datasets analyzed during the present study are available from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/) and The Gene Expression Omnibus (GEO) database of the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/geo/).
3.
Results
3.1. Identification of DEGs and common DEGs in three common cancers and COVID-19
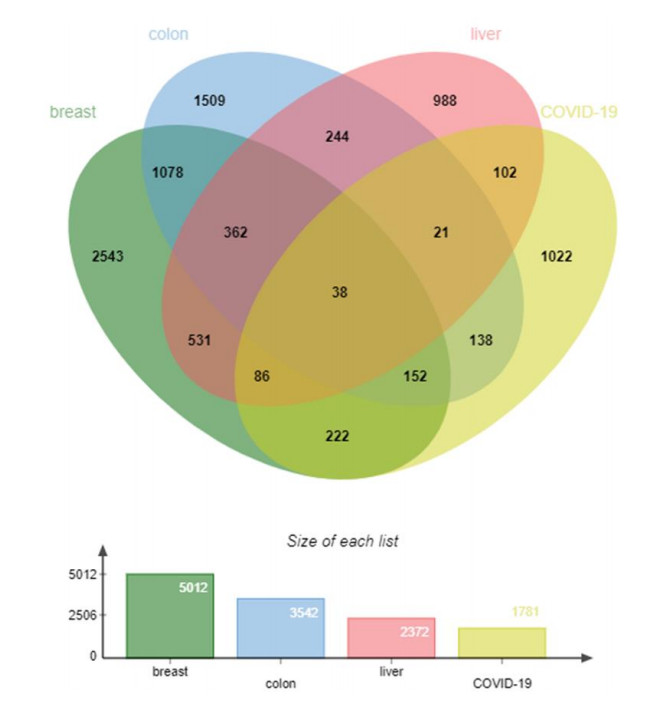
In this research, 1,781 genes were found to have a differential expression for COVID-19, where 391 were up-modulated and 1390 were down-modulated. From the assessment of TCGA data, 5012 DEGs (1997 up-modulated and 3015 down-modulated) were discovered in the breast cancer dataset, 3542 DEGs (1374 up-modulated and 2168 down-modulated) in the colon cancer data, and 2372 DEGs (979 up-modulated and 1393 down-modulated) in the liver cancer data. All of the significant DEGs were identified based on a p-value < 0.05 and |logFC| ≥ 1. Table 1 provides a summary of the information included within the datasets. Following the execution of the cross-comparison evaluation on Jvenn, 38 common DEGs were discovered from breast cancer, colon cancer, liver cancer, and COVID-19 datasets. Further analysis of these common genes was performed. All showed that all three cancers are linked to COVID-19 since they share one or more common genes. Figure 2 depicts the cumulative comparison study of the four datasets, as well as the extraction of the common DEGs.
3.2. Analyses of GO and KEGG enrichment
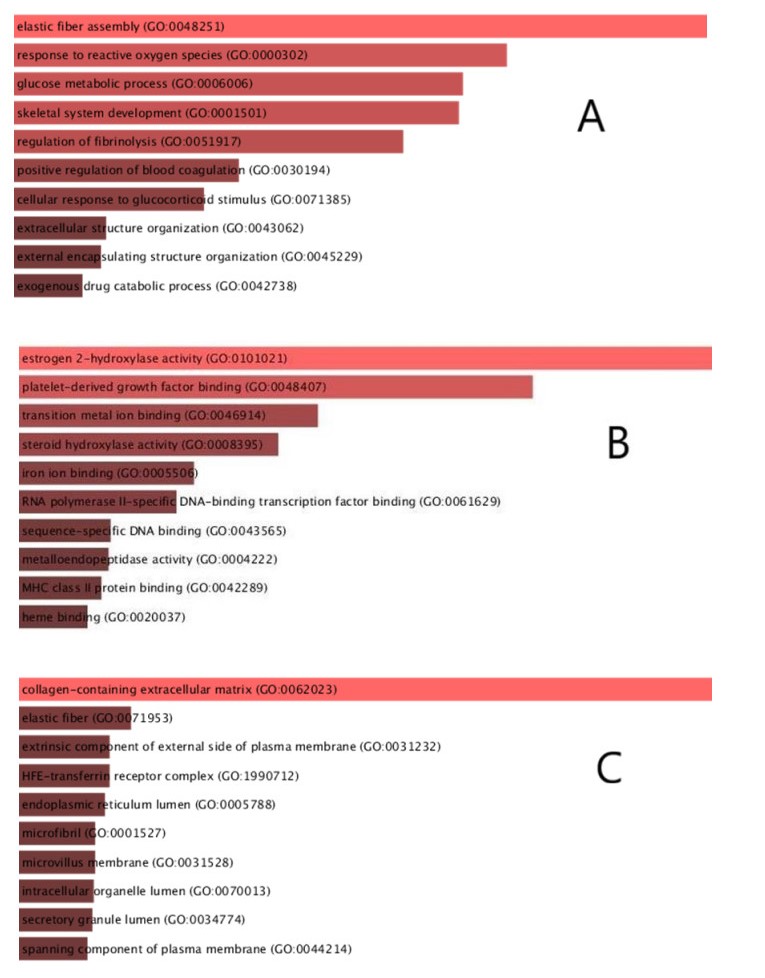
Enrichr was used to conduct GO and KEGG enrichment analyses of the common DEGs in order to determine their biological value and the enriched pathways behind them. The GO analysis was determined within three categories (BP, CC and MF). As a source for annotating data, the GO database was used. Table 2 summarizes the top ten terms in the BP, MF, and CC categories. The bar graph in Figure 3 demonstrates the complete ontological analysis in a linear manner for each of the different categories.
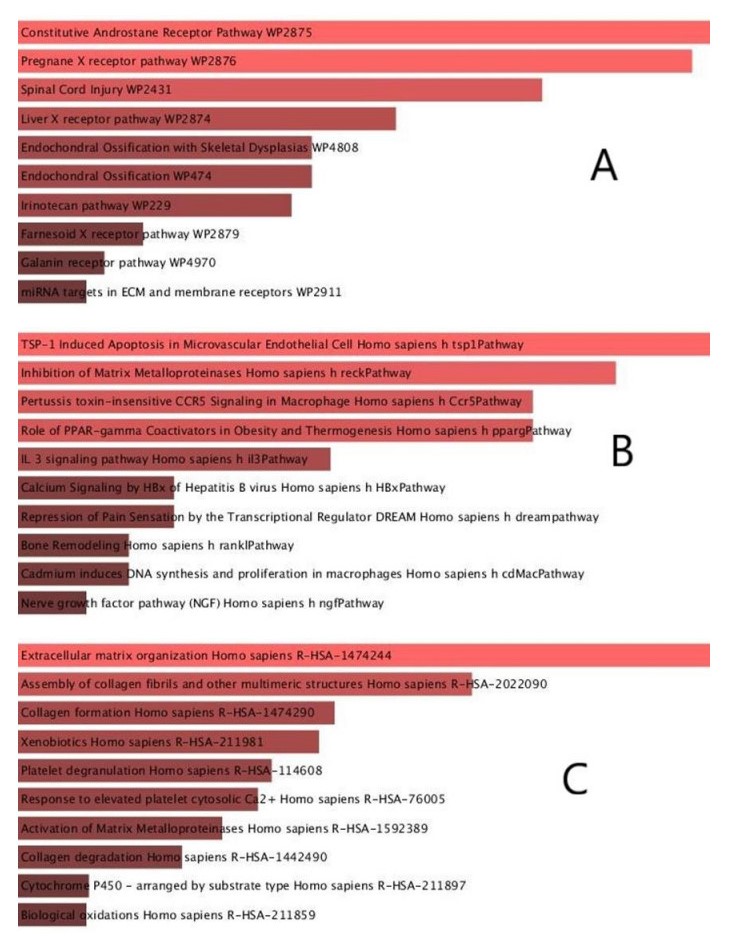
It was discovered via a pathway analysis that the organism has a reaction with its inherent alterations. In order to identify the most significantly altered pathways of the common DEGs in breast cancer, colon cancer, liver cancer, and COVID-19, four universal databases were used, including Reactome, WikiPathways, KEGG and Biocarta. Table 3 contains a summary of the most important pathways discovered via the analysis of the specified datasets. Furthermore, the pathway enrichment study is depicted in the bar charts in Figure 4.
3.3. Hub protein and submodule classification
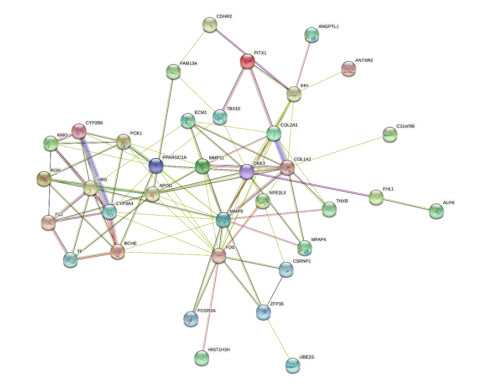
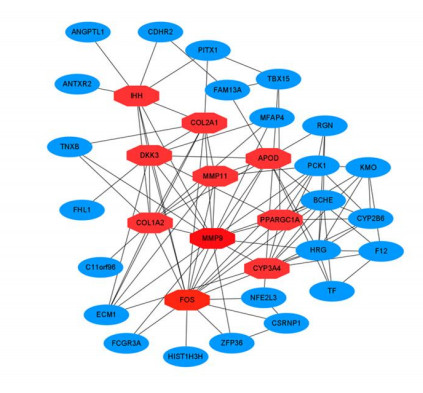
We examined the PPI network constructed from STRING and displayed it in Cytoscape in order to predict the interactions and adhesion routes of frequent DEGs. Figure 5 depicts the PPI network of common DEGs, which comprises 101 edges and 38 nodes. In a PPI network, the nodes with the most interconnections are interpreted to be hub genes. The topmost 10 DEGs were determined to be the most significant genes based on the PPI network analysis performed in Cytoscape utilizing the Cytohubba plugin. These hub genes comprised MMP9, FOS, COL1A2, COL2A1, DKK3, IHH, CYP3A4, PPARGC1A, MMP11 and APOD. The identified hub genes could be possible biomarkers for illnesses under investigation, and they may potentially lead to the development of novel treatment techniques. With this potential in mind, a submodule network was constructed using the Cytohubba plugin to gain a greater comprehension of the close interaction and vicinity of the hub genes. Figure 6 depicts the extended network of hub-gene connections resulting from the PPI network.
3.4. Identification of modulatory signatures
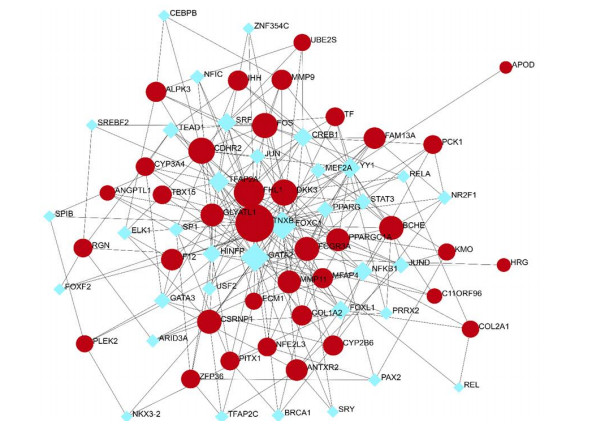
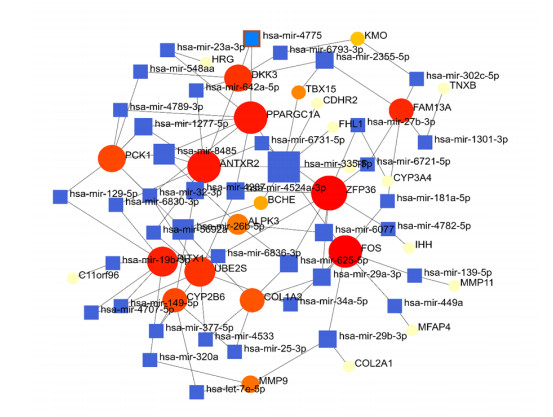
A network-based technique was used to decipher the modulatory TFs and miRNAs in order to discover significant modifications occurring at the transcription level and to gain knowledge of the regulatory molecules of hub genes or common DEGs. Figure 7 depicts the interplay of TFs and regulators with the common DEGs. Furthermore, Figure 8 depicts the interconnections of miRNA regulators with the common DEGs. Following the investigation of the TFs-gene and miRNAs-gene interactions networks, it was discovered that 72 TFs and 67 post-transcription (miRNAs) modulatory signatures interact with more than one common DEG. This simply suggests that there is a significant degree of crosstalk between them.
3.5. Candidate drug identification
We then evaluated PDI in order to comprehend the structural characteristics that are indicated for the sensitivity of receptors. In the case of common DEGs as prospective therapeutic targets in cancer and COVID-19, Enrichr was used to identify ten candidate medicinal compounds that were on the basis of transcriptomic profiles from the DSigDB. The ten leading chemical substances were determined based on their p-values, and they were then extracted. These possible medications have been proposed as therapies for the common DEGs seen in cancer and COVID-19. Table 4 contains the drugs that are successful for these common DEGs that have been identified in the DSigDB database.
4.
Discussion
The 2019 global pandemic of SARS-CoV-2 has become an emergency of major international concern. Growing evidence suggests that cancer patients have a greater vulnerability to this disease. In addition to having a greater susceptibility to this infection, cancer patients have a higher likelihood of advancing to a severe stage of SARS-CoV-2 than the general population[16,17]. Cancer is recognized as a pandemic, with over 18,000,000 individuals being diagnosed each year throughout the globe. The most comprehensive analysis from China includes data from 13,077 COVID-19 patients, among which 232 were found to develop cancer [18]. As a result, there will be more and more cancer patients developing SARS-CoV-2 infections in the years and decades to come. An increasing number of viruses and bacteria have been associated with human cancers [19]. To date, seven classes of viruses (Human papilloma virus (HPV), Hepatitis B virus (HBV), Hepatitis C virus (HCV), Kaposi's sarcoma associated herpesvirus (KSHV), Human T lymphotropic virus (HTLV), Merkel cell polyomavirus (MCpyV), Epstein Barr virus (EBV) have been identified to have oncogenic potential [20]. The oncoproteins of human tumor viruses regularly interact with the cellular epigenetic machinery. Such interactions alter the epigenome of the host cell and reprogram its gene expression pattern [21]. Helicobacter pylori is a bacterium known to be associated with gastroduodenal diseases such as chronic active gastritis, peptic ulcers, and gastric malignancies. Gene expression profiling in human gastric mucosa infected with Helicobacter pylori shows that host genes are dysregulated after Helicobacter pylori infection [22]. Some reports have studied the relationship between SARS-CoV-1 virus with the incidence of cancer. This virus which shares a lot of similarities with the SARS-CoV-2 virus has been reported to interfere with various signaling pathways associated with carcinogenic transformation of cells [23]. The genomic alterations of six SARS-CoV-2 receptor-related regulators (transmembrane serine protease 2 (TMPRSS2), angiotensinogen (AGT), angiotensin-converting enzyme 1 (ACE1), solute carrier family 6 member 19 (SLC6A19), angiotensin-converting enzyme 2 (ACE2), and angiotensin Ⅱ receptor type 2 (AGTR2)) and their clinical relevance across a broad spectrum of solid tumors were evaluated across 33 cancers [24]. Furthermore, four major similar signaling pathway, have been identified at the intersection of COVID-19 and cancer; namely, cytokine, type Ⅰ interferon (IFN-I), androgen receptor (AR), and immune checkpoint signaling [25]. In biomedical and system biology studies, expression profiling leveraging high throughput sequencing datasets is utilized to find biological marker candidates for various disorders [26]. In recent times, the capacity to analyze gene fusion, mutations/single nucleotide polymorphisms (SNPs), posttranscriptional alterations, and gene expression variations in diverse sets of therapies has been enhanced by RNA sequencing, a next-generation sequencing approach [27]. In this research, the transcriptome of three prevalent malignancies, as well as COVID-19, found that 38 common DEGs had comparable expression profiles. GO, and pathway enrichment studies on the basis of p-values were used to assess the biological significance of these common DEGs in order to comprehend the pathophysiology of four disorders. The GO analysis mainly included a biological process (BP, molecular activity), a cell component (CC, gene regulation function), and a molecular function (MF, molecular level activity). The common DEGs were mainly enriched in "elastic fiber assembly" (BP), "collagen-containing extracellular matrix" (CC), and "estrogen 2-hydroxylase activity" (MF). Furthermore, the analytical findings from KEGG enrichment illustrated the predominant involvement of common DEGs in the "ECM-receptor interaction", "relaxin signaling pathway", and "PI3K-Akt signaling pathway". Exploring these critical pathways could aid in the advancement of our knowledge of COVID-19 and the genesis of cancer. By using a network-based method, this research investigated the gene expression profiles in four RNA sequencing datasets of three common cancer types and COVID-19 patients. By analyzing patients the common phenotypic changes of cancer and COVID-19 patients, we hope to explore potential causative factors, and provide a theoretical basis for further study of these two diseases.
It is believed that a scarcity of specific medications is one of the primary causes of the continuing COVID-19 pandemic. We discovered the molecular targets that could be viable biological markers of cancer patients with SARS-CoV-2. A PPI network was constructed with the help of the DEG genes in order to truly comprehend the biological features of proteins and to explore therapeutic targets. In this study, 10 hub genes (MMP9, FOS, COL1A2, COL2A1, DKK3, IHH, CYP3A4, PPARGC1A, MMP11 and APOD) were examined, most of which were confirmed to be linked to the onset and progression of tumors in previous studies. Matrix metalloproteinases (MMPs) are endopeptidases with zinc (Zn2þ) as a cofactor that are found inside the cell and are bound by a biological membrane. MMP9 and MMP11 are two different forms of matrix metalloproteinases [28]. MMP-9 overexpression has been linked to a variety of malignancies, including breast, liver, and colon cancers [29,30,31,32]. Metallopeptidase activity affects respiratory disorders, including pulmonary fibrosis, acute lung injury, and acute respiratory distress syndrome (ARDS). MMP9 was previously found to be a significant biological marker of respiratory failure and SARS-CoV-2 infection in a prior investigation [33]. The FOS gene, which is found on chromosome 14q21–31 in humans, is responsible for encoding the nuclear oncoprotein c-Fos, the c-Fos protein binds to the c-Jun protein to generate a heterodimer (AP-1) that is capable of activating transcriptional activity. This is strongly linked to the proliferation and differentiation of cells, both of which contribute substantially to tumor transformation and reversal [34,35,36]. In the ECM-receptor pathway, the collagen type Ⅰ alpha 2 chain (COL1A2) performs a vital function and has been directly linked to the occurrence of diverse human malignancies [37]. DKK3 is a gene that inhibits tumor growth and its expression level has been shown to decrease in many kinds of cancers. DKK3 is a member of the DKK family (DKK1, DKK2, DKK3 and DKK4). It encodes for produced glycoproteins that are evolutionarily preserved and are made up of two unique cysteine-rich sites and act as an inhibitor of the oncogenic Wnt signaling pathway [38].The activation of the canonical Wnt/-catenin pathway in ARDS patients, particularly those with SARS-CoV-2, has been demonstrated to be linked to inflammatory and a cytokine storm, according to recent research [39,40]. However, whether the Wnt signaling pathway upregulation is the underlying molecular mechanism of cancer in patients with severe SARS-CoV-2 requires further investigation. Patients with lung adenocarcinoma and squamous cell carcinoma who had positive IHH expression had considerably shorter survival duration as opposed to those who had negative IHH expression, indicating that IHH is a prognostic factor for unsatisfactory prognosis [41]. CYP3A4, a member of the cytochrome P450 enzyme, is responsible for the metabolism of a wide range of anticancer drugs. Cancer patients with SARS-CoV-2 often require combination therapy, which can potentially lead to DDIs that result in an increased risk of side effects/toxicity or reduced effectiveness [42].Recently, Zulkar Naind et al. confirmed that PPARGC1A is a core protein implicated in SARS-CoV-2 infection as well as the risk factors associated with the disease [43].The study has shown that gene expression changes after infection with the SARS-CoV-2 [44]. Therefore, the hub genes that have been found may be used as prospective biological markers or, if the biological knowledge gained from SARS-CoV-2 is verified, as a new pharmacological target.
Moreover, TFs modulate the transcriptional ratio, whereas miRNA is a critical participant in RNA knockdown and gene expression control at the post-transcriptional level. As a result, both are necessary for understanding the progression mechanism of the illness. This research discovered links between the DEGs that were studied and their corresponding TFs and modulatory miRNAs. Several TFs were discovered, including STAT3, NFKB1, FOXC1, HINFP and JUN. There is evidence that these TFs are associated with viral-induced acute respiratory illnesses or the genesis and progression of malignancies [45,46,47,48]. Furthermore, several miRNAs play a role in breast cancer, (e.g. hsa-mir-335-5p, hsa-mir-139-5p, hsa-mir-25-3p, hsa-miR-32-3p) [49,50], liver cancer (e.g.hsa-mir-139-5p, hsa-mir-25-3p) [51,52], and colon cancer (e.g. hsa-mir-1301-3p, hsa-mir-29b-3p, hsa-mir-25-3p, hsa-mir-129-5p, hsa-miR-1277-5p) [53,54,55,56,57].There are several miRNAs involved in coronaviruses infection; for example, miRNAs were highly expressed in MERS-CoV-infected cells and may play important roles in disease development [58]. hsa-miR-139-5p can act as a risk factor for severe COVID-19 [59]. However, the role of miRNA in tumor patients with SARS-CoV-2 infection requires further research.
As per the latest report from WHO, no appropriate therapies or medicines for preventing and treating COVID-19 have been established and approved to date [2]. However, for years, several institutions, government agencies, research institutes, and pharmaceuticals have conducted clinical and preclinical studies to determine whether current medication moieties are effective in treating the disease on the basis of their prior experience with viral infections. In in vitro cell cultures, Wang et al. demonstrated that two medicines, remdesivir and chloroquine, were successful in managing SARS-CoV-2 infection [60]. Additionally, a clinical experiment revealed that the antibiotics azithromycin and hydroxychloroquine had a substantial impact on SARS-CoV-2 by interfering with its genome replication [61,62]. In this study, we identified several potential drugs to treat SARS-CoV-2 infection, such as lycorine and astemizole. Lycorine is a natural alkaloid that has been derived from the lycoris plant and has been shown to have a number of biological effects, such as antitumor, anti-viral, and anti-malaria activities [63,64]. Similarly, studies have shown that quartzine can reduce acute lung injury [65]. New research shows that astemizole may suppress the invasion of SARS-CoV-2 spike pseudoviruses by binding to the ACE2 receptor [66]. Therefore, these drugs are potential candidates for anti-coronavirus therapy.
5.
Conclusions
In this study, the common differential genes of three different cancer types and COVID-19 patients were obtained through bioinformatics techniques. Additionally, the top 10 target genes were obtained through a PPI analysis of common differential genes. These target genes may be biomarkers for cancer or SARS-CoV-2 infection, which translates to potential drug targets. The association between these target genes and TFs and miRNAs was also studied to better understand their role in the occurrence and development of disease. Meanwhile, several targeted drugs with potential clinical value were identified for treating SARS-CoV-2 infection in patients with common types of cancer. Finally, our study uses public databases and bioinformatics analysis to find common genotype changes in cancer and COVID-19, but whether these genotype changes are potential causative factors of the two diseases, these potential gene targets and drugs can be further used in the clinic needs to be verified through experimental studies such as cells and animal models. This is the biggest deficiency in our research, and it is also the focus of our follow-up further research.
Acknowledgments
We would like to acknowledge the TCGA and GEO for providing data. This research received no external funding.
Conflict of interest
All authors declare no conflicts of interest in this paper.









 DownLoad:
DownLoad: