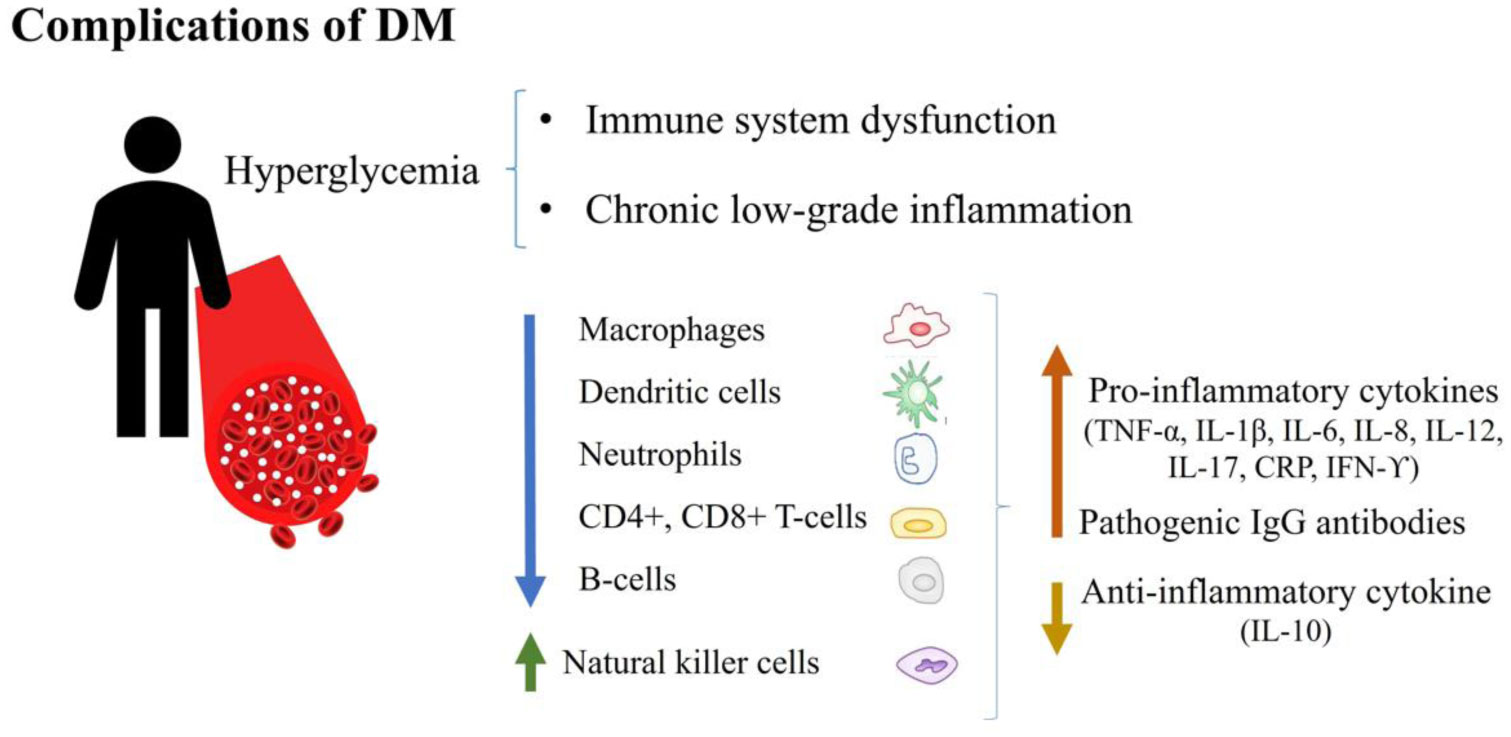
|
[1]
|
Asselah T, Durantel D, Pasmant E, et al. (2021) COVID-19: discovery, diagnostics and drug development. J Hepatol 74: 168-184. doi: 10.1016/j.jhep.2020.09.031

|
|
[2]
|
Rojas R, Basto A, Aguilar C, et al. (2018) Prevalencia de diabetes por diagnóstico médico previo en México. Salud Publica Mex 60: 224-232. doi: 10.21149/8566

|
|
[3]
|
Levaillant M, Lièvre G, Baert G (2019) Ending diabetes in Mexico. Lancet 394: 467-468. doi: 10.1016/S0140-6736(19)31662-9

|
|
[4]
|
Singer M (2020) Deadly companions: COVID-19 and diabetes in Mexico. Med Anthropol 39: 660-665. doi: 10.1080/01459740.2020.1805742

|
|
[5]
|
Zhang W, Davis BD, Chen SS, et al. (2021) Emergence of a novel SARS-CoV-2 variant in southern California. JAMA 325: 1324-1326. doi: 10.1001/jama.2021.1612

|
|
[6]
|
Zucman N, Uhel F, Descamps D, et al. (2021) Severe reinfection with South African severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant 501Y.V2: a case report. Clin Infect Dis ciab129. doi: 10.1093/cid/ciab129

|
|
[7]
|
Fujino T, Nomoto H, Kutsuna S, et al. (2021) Novel SARS-CoV-2 variant in travelers from Brazil to Japan. Emerg Infect Dis 27: 1243-1245. doi: 10.3201/eid2704.210138

|
|
[8]
|
Yadav PD, Nyayanit DA, Sahay RR, et al. (2021) Isolation and characterization of the new SARS-CoV-2 variant in travellers from the United Kingdom to India: VUI-202012/01 of the B.1.1.7 lineage. J Travel Med 28: taab009. doi: 10.1093/jtm/taab009

|
|
[9]
|
Zuckerman NS, Fleishon S, Bucris E, et al. (2021) A unique SARS-CoV-2 spike protein P681H variant detected in Israel. Vaccines 9: 616. doi: 10.3390/vaccines9060616

|
|
[10]
|
Munitz A, Yechezkel M, Dickstein Y, et al. (2021) The rise of SARS-CoV-2 variant B.1.1.7 in Israel intensifies the role of surveillance and vaccination in elderly. medRxiv 2021.
|
|
[11]
|
Mor O, Zuckerman NS, Hazan I, et al. (2021) BNT162b2 vaccination efficacy is marginally affected by the SARS-CoV-2 B.1.351 variant in fully vaccinated individuals. Avaliable at SSRN 3878825 .
|
|
[12]
|
Munitz A, Yechezkel M, Dickstein Y, et al. (2021) Report BNT162b2 vaccination effectively prevents the rapid populations in Israel ll BNT162b2 vaccination effectively prevents the rapid rise of SARS-CoV-2 variant B.1.1.7 in high-risk populations in Israel. Cell Rep Med 2: 100264. doi: 10.1016/j.xcrm.2021.100264

|
|
[13]
|
Haas EJ, Angulo FJ, Mclaughlin JM, et al. (2021) Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 397: 1819-1829. doi: 10.1016/S0140-6736(21)00947-8

|
|
[14]
|
Kustin T, Harel N, Finkel U, et al. (2021) Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med 27: 1379-1384. doi: 10.1038/s41591-021-01413-7

|
|
[15]
|
Hu T, Liu Y, Zhao M, et al. (2020) A comparison of COVID-19, SARS and MERS. PeerJ 8: e9725. doi: 10.7717/peerj.9725

|
|
[16]
|
Harrison AG, Lin T, Wang P (2020) Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol 41: 1100-1115. doi: 10.1016/j.it.2020.10.004

|
|
[17]
|
Yuki K, Fujiogi M, Koutsogiannaki S (2020) COVID-19 pathophysiology: a review. Clin Immunol 215: 108427. doi: 10.1016/j.clim.2020.108427

|
|
[18]
|
Sardu C, Gambardella J, Morelli MB, et al. (2020) Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J Clin Med 9: 1417. doi: 10.3390/jcm9051417

|
|
[19]
|
Safwat MA, Abdul-Rahman A, Forsan H, et al. (2021) COVID-19; Immunology, pathology, severity and immunosuppressants. Azhar Int J Pharm Med Sci 1: 1-14. doi: 10.21608/aijpms.2021.50103.1005

|
|
[20]
|
Kabashneh S, Ali H, Alkassis S (2020) Multi-Organ failure in a patient with diabetes due to COVID-19 with clear lungs. Cureus 12: e8147.
|
|
[21]
|
Guo L, Shi Z, Zhang Y, et al. (2020) Comorbid diabetes and the risk of disease severity or death among 8807 COVID-19 patients in China: a meta-analysis. Diabetes Res Clin Pract 166: 108346. doi: 10.1016/j.diabres.2020.108346

|
|
[22]
|
Zhou Y, Chi J, Lv W, et al. (2021) Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19). Diabetes Metab Res Rev 37: e3377.
|
|
[23]
|
Parveen R, Sehar N, Bajpai R, et al. (2020) Association of diabetes and hypertension with disease severity in covid-19 patients: a systematic literature review and exploratory meta-analysis. Diabetes Res Clin Pract 166: 108295. doi: 10.1016/j.diabres.2020.108295

|
|
[24]
|
Frydrych LM, Bian G, O'Lone DE, et al. (2018) Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J Leukoc Biol 104: 525-534. doi: 10.1002/JLB.5VMR0118-021RR

|
|
[25]
|
Sur J, Sharma J, Sharma D (2021) Diabetes might augment the severity of COVID-19: a current prospects. Front Cardiovasc Med 7: 613255. doi: 10.3389/fcvm.2020.613255

|
|
[26]
|
Kumar A, Arora A, Sharma P, et al. (2020) Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr 14: 535-545. doi: 10.1016/j.dsx.2020.04.044

|
|
[27]
|
Jeong IK, Yoon KH, Lee MK (2020) Diabetes and COVID-19: global and regional perspectives. Diabetes Res Clin Pract 166: 108303. doi: 10.1016/j.diabres.2020.108303

|
|
[28]
|
Chen Y, Yang D, Cheng B, et al. (2020) Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care 43: 1399-1407. doi: 10.2337/dc20-0660

|
|
[29]
|
Mantovani A, Byrne CD, Zheng MH, et al. (2020) Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis 30: 1236-1248. doi: 10.1016/j.numecd.2020.05.014

|
|
[30]
|
Du M, Lin YX, Yan WX, et al. (2020) Prevalence and impact of diabetes in patients with COVID-19 in China. World J Diabetes 11: 468-480. doi: 10.4239/wjd.v11.i10.468

|
|
[31]
|
Targher G, Mantovani A, Wang XB, et al. (2020) Patients with diabetes are at higher risk for severe illness from COVID-19. Diabetes Metab 46: 335-337. doi: 10.1016/j.diabet.2020.05.001

|
|
[32]
|
Ciardullo S, Zerbini F, Perra S, et al. (2020) Impact of diabetes on COVID-19-related in-hospital mortality: a retrospective study from Northern Italy. J Endocrinol Invest 44: 843-850. doi: 10.1007/s40618-020-01382-7

|
|
[33]
|
Halvatsiotis P, Kotanidou A, Tzannis K, et al. (2020) Demographic and clinical features of critically ill patients with COVID-19 in Greece: the burden of diabetes and obesity. Diabetes Res Clin Pract 166: 108331. doi: 10.1016/j.diabres.2020.108331

|
|
[34]
|
Nachtigall I, Lenga P, Jóźwiak K, et al. (2020) Clinical course and factors associated with outcomes among 1904 patients hospitalized with COVID-19 in Germany: an observational study. Clin Microbiol Infect 26: 1663-1669. doi: 10.1016/j.cmi.2020.08.011

|
|
[35]
|
Sutter W, Duceau B, Vignac M, et al. (2020) Association of diabetes and outcomes in patients with COVID-19: propensity score-matched analyses from a French retrospective cohort. Diabetes Metab 47: 101222. doi: 10.1016/j.diabet.2020.101222

|
|
[36]
|
Barron E, Bakhai C, Kar P, et al. (2020) Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol 8: 813-822. doi: 10.1016/S2213-8587(20)30272-2

|
|
[37]
|
Ge Y, Sun S, Shen Y (2021) Estimation of case-fatality rate in COVID-19 patients with hypertension and diabetes mellitus in the New York State: a preliminary report. Epidemiol Infect 149: e14. doi: 10.1017/S0950268821000066

|
|
[38]
|
Hussain S, Baxi H, Chand Jamali M, et al. (2020) Burden of diabetes mellitus and its impact on COVID-19 patients: a meta-analysis of real-world evidence. Diabetes Metab Syndr 14: 1595-1602. doi: 10.1016/j.dsx.2020.08.014

|
|
[39]
|
Ashktorab H, Pizuorno A, Oskroch G, et al. (2021) COVID-19 in Latin America: symptoms, morbidities, and gastrointestinal manifestations. Gastroenterology 160: 938-940. doi: 10.1053/j.gastro.2020.10.033

|
|
[40]
|
Izzi-Engbeaya C, Distaso W, Amin A, et al. (2021) Adverse outcomes in COVID-19 and diabetes: a retrospective cohort study from three London teaching hospitals. BMJ Open Diabetes Res Care 9: e001858. doi: 10.1136/bmjdrc-2020-001858

|
|
[41]
|
Ebekozien O, Agarwal S, Noor N, et al. (2020) Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab 106: e1755-e1762. doi: 10.1210/clinem/dgaa920

|
|
[42]
|
Hafidh K, Abbas S, Khan A, et al. (2020) The clinical characteristics and outcomes of COVID-19 infections in patients with diabetes at a tertiary care center in the UAE. Dubai Diabetes Endocrinol J 26: 158-163. doi: 10.1159/000512232

|
|
[43]
|
Yan Y, Yang Y, Wang F, et al. (2020) Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care 8: e001343. doi: 10.1136/bmjdrc-2020-001343

|
|
[44]
|
Ling P, Luo S, Zheng X, et al. (2021) Elevated fasting blood glucose within the first week of hospitalization was associated with progression to severe illness of COVID-19 in patients with preexisting diabetes: a multicenter observational study. J Diabetes 13: 89-93. doi: 10.1111/1753-0407.13121

|
|
[45]
|
Silverio A, Di Maio M, Citro R, et al. (2021) Cardiovascular risk factors and mortality in hospitalized patients with COVID-19: systematic review and meta-analysis of 45 studies and 18,300 patients. BMC Cardiovasc Disord 21: 23. doi: 10.1186/s12872-020-01816-3

|
|
[46]
|
Acharya D, Lee K, Lee DS, et al. (2020) Mortality rate and predictors of mortality in hospitalized COVID-19 patients with diabetes. Healthcare 8: 338. doi: 10.3390/healthcare8030338

|
|
[47]
|
Bode B, Garrett V, Messler J, et al. (2020) Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol 14: 813-821. doi: 10.1177/1932296820924469

|
|
[48]
|
Bhandari S, Rankawat G, Singh A, et al. (2020) Impact of glycemic control in diabetes mellitus on management of COVID-19 infection. Int J Diabetes Dev Ctries 1-6.
|
|
[49]
|
Ceriello A, De Nigris V, Prattichizzo F (2020) Why is hyperglycaemia worsening COVID-19 and its prognosis? Diabetes Obes Metab 22: 1951-1952. doi: 10.1111/dom.14098

|
|
[50]
|
Lee MH, Wong C, Ng CH, et al. (2021) Effects of hyperglycaemia on complications of COVID-19: a meta-analysis of observational studies. Diabetes Obes Metab 23: 287-289. doi: 10.1111/dom.14184

|
|
[51]
|
Singh AK, Singh R (2020) Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Diabetes Res Clin Pract 167: 108382. doi: 10.1016/j.diabres.2020.108382

|
|
[52]
|
Carrasco-Sánchez FJ, López-Carmona MD, Martínez-Marcos FJ, et al. (2021) Admission hyperglycaemia as a predictor of mortality in patients hospitalized with COVID-19 regardless of diabetes status: data from the Spanish SEMI-COVID-19 Registry. Ann Med 53: 103-116. doi: 10.1080/07853890.2020.1836566

|
|
[53]
|
Wang S, Ma P, Zhang S, et al. (2020) Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia 63: 2102-2111. doi: 10.1007/s00125-020-05209-1

|
|
[54]
|
Li H, Tian S, Chen T, et al. (2020) Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes Metab 22: 1897-1906. doi: 10.1111/dom.14099

|
|
[55]
|
Lim S, Bae JH, Kwon HS, et al. (2021) COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol 17: 11-30. doi: 10.1038/s41574-020-00435-4

|
|
[56]
|
Abu-Farha M, Al-Mulla F, Thanaraj TA, et al. (2020) Impact of diabetes in patients diagnosed with COVID-19. Front Immunol 11: 576818. doi: 10.3389/fimmu.2020.576818

|
|
[57]
|
Yaribeygi H, Sathyapalan T, Jamialahmadi T, et al. (2020) The impact of diabetes mellitus in COVID-19: a mechanistic review of molecular interactions. J Diabetes Res 2020: 5436832.
|
|
[58]
|
Singh AK, Gupta R, Ghosh A, et al. (2020) Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr 14: 303-310. doi: 10.1016/j.dsx.2020.04.004

|
|
[59]
|
Erener S (2020) Diabetes, infection risk and COVID-19. Mol Metab 39: 101044. doi: 10.1016/j.molmet.2020.101044

|
|
[60]
|
Azar WS, Njeim R, Fares AH, et al. (2020) COVID-19 and diabetes mellitus: how one pandemic worsens the other. Rev Endocr Metab Disord 21: 451-463. doi: 10.1007/s11154-020-09573-6

|
|
[61]
|
Banik GR, Alqahtani AS, Booy R, et al. (2016) Risk factors for severity and mortality in patients with MERS-CoV: analysis of publicly available data from Saudi Arabia. Virol Sin 31: 81-84. doi: 10.1007/s12250-015-3679-z

|
|
[62]
|
Kulcsar KA, Coleman CM, Beck SE, et al. (2019) Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight 4: e131774. doi: 10.1172/jci.insight.131774

|
|
[63]
|
Halaji M, Farahani A, Ranjbar R, et al. (2020) Emerging coronaviruses: first SARS, second MERS and third SARS-COV-2: epidemiological updates of COVID-19. Infez Med 28: 6-17.
|
|
[64]
|
Muniyappa R, Gubbi S (2020) COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab 318: E736-E741. doi: 10.1152/ajpendo.00124.2020

|
|
[65]
|
Petersmann A, Nauck M, Müller-Wieland D, et al. (2018) Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes 127: S1-S7.
|
|
[66]
|
Punthakee Z, Goldenberg R, et al. (2018) Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes 42: S10-S15. doi: 10.1016/j.jcjd.2017.10.003

|
|
[67]
|
Daryabor G, Atashzar MR, Kabelitz D, et al. (2020) The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front Immunol 11: 1582. doi: 10.3389/fimmu.2020.01582

|
|
[68]
|
Papatheodorou K, Banach M, Bekiari E, et al. (2018) Complications of diabetes 2017. J Diabetes Res 2018: 3086167. doi: 10.1155/2018/3086167

|
|
[69]
|
Huang D, Refaat M, Mohammedi K, et al. (2017) Macrovascular complications in patients with diabetes and prediabetes. Biomed Res Int 2017: 7839101.
|
|
[70]
|
Khalil H (2017) Diabetes microvascular complications – A clinical update. Diabetes Metab Syndr 11: S133-S139. doi: 10.1016/j.dsx.2016.12.022

|
|
[71]
|
Struijs JN, Baan CA, Schellevis FG, et al. (2006) Comorbidity in patients with diabetes mellitus: impact on medical health care utilization. BMC Health Serv Res 6: 84. doi: 10.1186/1472-6963-6-84

|
|
[72]
|
Prattichizzo F, de Candia P, Nicolucci A, et al. (2021) Elevated HbA1c levels in pre-Covid-19 infection increases the risk of mortality: a sistematic review and meta-analysis. Diabetes Metab Res Rev e3476.
|
|
[73]
|
Mirani M, Favacchio G, Carrone F, et al. (2020) Impact of comorbidities and glycemia at admission and dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes with covid-19: a case series from an academic hospital in lombardy, Italy. Diabetes Care 43: 3042-3049. doi: 10.2337/dc20-1340

|
|
[74]
|
Orioli L, Servais T, Belkhir L, et al. (2021) Clinical characteristics and short-term prognosis of in-patients with diabetes and COVID-19: a retrospective study from an academic center in Belgium. Diabetes Metab Syndr 15: 149-157. doi: 10.1016/j.dsx.2020.12.020

|
|
[75]
|
Sun Y, Guan X, Jia L, et al. (2021) Independent and combined effects of hypertension and diabetes on clinical outcomes in patients with COVID-19: a retrospective cohort study of Huoshen Mountain Hospital and Guanggu Fangcang Shelter Hospital. J Clin Hypertens 23: 218-231. doi: 10.1111/jch.14146

|
|
[76]
|
Vieira E, Mirizio GG, Barin GR, et al. (2020) Clock genes, inflammation and the immune system—Implications for diabetes, obesity and neurodegenerative diseases. Int J Mol Sci 21: 9743. doi: 10.3390/ijms21249743

|
|
[77]
|
Zhou T, Hu Z, Yang S, et al. (2018) Role of adaptive and innate immunity in type 2 diabetes mellitus. J Diabetes Res 2018: 7457269.
|
|
[78]
|
Hodgson K, Morris J, Bridson T, et al. (2015) Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology 144: 171-185. doi: 10.1111/imm.12394

|
|
[79]
|
Alzaid F, Julla J, Diedisheim M, et al. (2020) Monocytopenia, monocyte morphological anomalies and hyperinflammation characterise severe COVID-19 in type 2 diabetes. EMBO Mol Med 12: e13038. doi: 10.15252/emmm.202013038

|
|
[80]
|
De Candia P, Prattichizzo F, Garavelli S, et al. (2019) Type 2 diabetes: how much of an autoimmune disease? Front Endocrinol (Lausanne) 10: 451. doi: 10.3389/fendo.2019.00451

|
|
[81]
|
Prattichizzo F, Giuliani A, Sabbatinelli J, et al. (2020) Prevalence of residual inflammatory risk and associated clinical variables in patients with type 2 diabetes. Diabetes Obes Metab 22: 1696-1700. doi: 10.1111/dom.14081

|
|
[82]
|
Maedler K, Spinas GA, Lehmann R, et al. (2001) Glucose induces β-Cell apoptosis via upregulation of the Fas receptor in human islets. Diabetes 50: 1683-1690. doi: 10.2337/diabetes.50.8.1683

|
|
[83]
|
Maedler K, Sergeev P, Ris F, et al. (2002) Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110: 851-860. doi: 10.1172/JCI200215318

|
|
[84]
|
Zhou W, Ye S, Wang W, et al. (2020) Clinical features of COVID-19 patients with diabetes and secondary hyperglycemia. J Diabetes Res 2020: 3918723.
|
|
[85]
|
Roganović J (2021) Downregulation of microRNA-146a in diabetes, obesity and hypertension may contribute to severe COVID-19. Med Hypotheses 146: 110448. doi: 10.1016/j.mehy.2020.110448

|
|
[86]
|
Gou L, Xiang M, Ran X, et al. (2021) Hyperosmolarity deserves more attention in critically Ill COVID-19 patients with diabetes: a cohort-based study. Diabetes Metab Syndr Obes Targets Ther 14: 47-58. doi: 10.2147/DMSO.S284148

|
|
[87]
|
Zheng M, Wang X, Guo H, et al. (2021) The cytokine profiles and immune response are increased in COVID-19 patients with type 2 diabetes mellitus. J Diabetes Res 2021: 9526701. doi: 10.1155/2021/9526701

|
|
[88]
|
Guo W, Li M, Dong Y, et al. (2020) Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev e3319.
|
|
[89]
|
Cheng Y, Yue L, Wang Z, et al. (2021) Hyperglycemia associated with lymphopenia and disease severity of COVID-19 in type 2 diabetes mellitus. J Diabetes Complications 35: 107809. doi: 10.1016/j.jdiacomp.2020.107809

|
|
[90]
|
Sun Y, Zhao R, Hu Z, et al. (2020) Differences in the clinical and hematological characteristics of COVID-19 patients with and without type 2 diabetes. J Diabetes Res 2020: 1038585.
|
|
[91]
|
Zhang Y, Li H, Zhang J, et al. (2020) The clinical characteristics and outcomes of patients with diabetes and secondary hyperglycaemia with coronavirus disease 2019: a single-centre, retrospective, observational study in Wuhan. Diabetes Obes Metab 22: 1443-1454. doi: 10.1111/dom.14086

|
|
[92]
|
Ulhaq ZS, Soraya GV (2020) Interleukin-6 as a potential biomarker of COVID-19 progression. Médecine Mal Infect 50: 382-383. doi: 10.1016/j.medmal.2020.04.002

|
|
[93]
|
Scheller J, Chalaris A, Schmidt-Arras D, et al. (2011) The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813: 878-888. doi: 10.1016/j.bbamcr.2011.01.034

|
|
[94]
|
Muangchan C, Pope JE (2013) The significance of interleukin-6 and C-reactive protein in systemic sclerosis: a systematic literature review. Clin Exp Rheumatol 31: 122-134.
|
|
[95]
|
Mishra Y, Pathak BK, Mohakuda SS, et al. (2020) Relation of D-dimer levels of COVID-19 patients with diabetes mellitus. Diabetes Metab Syndr 14: 1927-1930. doi: 10.1016/j.dsx.2020.09.035

|
|
[96]
|
Rostami M, Mansouritorghabeh H (2020) D-dimer level in COVID-19 infection: a systematic review. Expert Rev Hematol 13: 1265-1275. doi: 10.1080/17474086.2020.1831383

|
|
[97]
|
Mei H, Luo L, Hu Y (2020) Thrombocytopenia and thrombosis in hospitalized patients with COVID-19. J Hematol Oncol 13: 161. doi: 10.1186/s13045-020-01003-z

|
|
[98]
|
Zahran AM, El-Badawy O, Mohamad IL, et al. (2018) Platelet activation and platelet–leukocyte aggregates in type I diabetes mellitus. Clin Appl Thromb Hemost 24: 230S-239S. doi: 10.1177/1076029618805861

|
|
[99]
|
Kaur R, Kaur M, Singh J (2018) Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol 17: 121. doi: 10.1186/s12933-018-0763-3

|
|
[100]
|
Shang J, Wang Q, Zhang H, et al. (2021) The relationship between diabetes mellitus and COVID-19 prognosis: a retrospective cohort study in Wuhan, China. Am J Med 134: e6-e14. doi: 10.1016/j.amjmed.2020.05.033

|
|
[101]
|
Guo T, Shen Q, Ouyang X, et al. (2021) Clinical findings in diabetes mellitus patients with COVID-19. J Diabetes Res 2021: 7830136.
|
|
[102]
|
Al-Salameh A, Lanoix JP, Bennis Y, et al. (2021) Characteristics and outcomes of COVID-19 in hospitalized patients with and without diabetes. Diabetes Metab Res Rev 37: e3388. doi: 10.1002/dmrr.3388

|
|
[103]
|
Chen X, Hu W, Ling J, et al. (2020) Hypertension and diabetes delay the viral clearance in COVID-19 patients. medRxiv .
|
|
[104]
|
Clarke NE, Turner AJ (2012) Angiotensin-converting enzyme 2: the first decade. Int J Hypertens 2012: 307315. doi: 10.1155/2012/307315

|
|
[105]
|
Warner FJ, Smith AI, Hooper NM, et al. (2004) Angiotensin-converting enzyme-2: a molecular and cellular perspective. Cell Mol Life Sci 61: 2704-2713. doi: 10.1007/s00018-004-4240-7

|
|
[106]
|
Santos RAS, Ferreira AJ, Simões E, Silva AC (2008) Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exp Physiol 93: 519-527. doi: 10.1113/expphysiol.2008.042002

|
|
[107]
|
Santos RAS, Ferreira AJ, Verano-Braga T, et al. (2013) Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. J Endocrinol 216: R1-R17. doi: 10.1530/JOE-12-0341

|
|
[108]
|
Hashimoto T, Perlot T, Rehman A, et al. (2012) ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487: 477-481. doi: 10.1038/nature11228

|
|
[109]
|
Perlot T, Penninger JM (2013) ACE2 – From the renin-angiotensin system to gut microbiota and malnutrition. Microbes Infect 15: 866-873. doi: 10.1016/j.micinf.2013.08.003

|
|
[110]
|
Hamming I, Timens W, Bulthuis MLC, et al. (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631-637. doi: 10.1002/path.1570

|
|
[111]
|
Tikellis C, Johnston CI, Forbes JM, et al. (2003) Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension 41: 392-397. doi: 10.1161/01.HYP.0000060689.38912.CB

|
|
[112]
|
Xia H, Lazartigues E (2008) Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem 107: 1482-1494. doi: 10.1111/j.1471-4159.2008.05723.x

|
|
[113]
|
Xu H, Zhong L, Deng J, et al. (2020) High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 12: 8. doi: 10.1038/s41368-020-0074-x

|
|
[114]
|
To KF, Lo AWI (2004) Exploring the pathogenesis of severe acute respiratory syndrome (SARS): The tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2). J Pathol 203: 740-743. doi: 10.1002/path.1597

|
|
[115]
|
Batlle D, Wysocki J, Satchell K (2020) Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci 134: 543-545. doi: 10.1042/CS20200163

|
|
[116]
|
Ni W, Yang X, Yang D, et al. (2020) Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care 24: 422. doi: 10.1186/s13054-020-03120-0

|
|
[117]
|
Zhang H, Penninger JM, Li Y, et al. (2020) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46: 586-590. doi: 10.1007/s00134-020-05985-9

|
|
[118]
|
Shang J, Wan Y, Luo C, et al. (2020) Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A 117: 11727-11734. doi: 10.1073/pnas.2003138117

|
|
[119]
|
Valencia I, Peiró C, Lorenzo Ó, et al. (2020) DPP4 and ACE2 in diabetes and COVID-19: therapeutic targets for cardiovascular complications? Front Pharmacol 11: 1161. doi: 10.3389/fphar.2020.01161

|
|
[120]
|
Bourgonje AR, Abdulle AE, Timens W, et al. (2020) Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol 251: 228-248. doi: 10.1002/path.5471

|
|
[121]
|
Chen J, Jiang Q, Xia X, et al. (2020) Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell 19: e13168.
|
|
[122]
|
Mizuiri S, Hemmi H, Arita M, et al. (2008) Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis 51: 613-623. doi: 10.1053/j.ajkd.2007.11.022

|
|
[123]
|
Reich HN, Oudit GY, Penninger JM, et al. (2008) Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int 74: 1610-1616. doi: 10.1038/ki.2008.497

|
|
[124]
|
Gilbert RE, Caldwell L, Misra PS, et al. (2021) Overexpression of the severe acute respiratory syndrome coronavirus-2 receptor, angiotensin-converting enzyme 2, in diabetic kidney disease: implications for kidney injury in novel coronavirus disease 2019. Can J Diabetes 45: 162-166.e1. doi: 10.1016/j.jcjd.2020.07.003

|
|
[125]
|
Soldo J, Heni M, Königsrainer A, et al. (2020) Increased hepatic ace2 expression in nafl and diabetes-a risk for covid-19 patients? Diabetes Care 43: e134-e136. doi: 10.2337/dc20-1458

|
|
[126]
|
Wijnant SRA, Jacobs M, Van Eeckhoutte HP, et al. (2020) Expression of ACE2, the SARS-CoV-2 receptor, in lung tissue of patients with type 2 diabetes. Diabetes 69: 2691-2699. doi: 10.2337/db20-0669

|
|
[127]
|
Parit R, Jayavel S (2021) Association of ACE inhibitors and angiotensin type II blockers with ACE2 overexpression in COVID-19 comorbidities: a pathway-based analytical study. Eur J Pharmacol 896: 173899. doi: 10.1016/j.ejphar.2021.173899

|
|
[128]
|
Pal R, Bhadada SK (2020) COVID-19 and diabetes mellitus: an unholy interaction of two pandemics. Diabetes Metab Syndr 14: 513-517. doi: 10.1016/j.dsx.2020.04.049

|
|
[129]
|
Menon R, Otto EA, Sealfon R, et al. (2020) SARS-CoV-2 receptor networks in diabetic and COVID-19–associated kidney disease. Kidney Int 98: 1502-1518. doi: 10.1016/j.kint.2020.09.015

|
|
[130]
|
Batchu SN, Kaur H, Yerra VG, et al. (2021) Lung and kidney ACE2 and TMPRSS2 in renin-angiotensin system blocker-treated comorbid diabetic mice mimicking host factors that have been linked to severe COVID-19. Diabetes 70: 759-771. doi: 10.2337/db20-0765

|
|
[131]
|
Sriram K, Insel PA (2020) Risks of ACE inhibitor and ARB usage in COVID-19: evaluating the evidence. Clin Pharmacol Ther 108: 236-241. doi: 10.1002/cpt.1863

|
|
[132]
|
Burrell LM, Harrap SB, Velkoska E, et al. (2013) The ACE2 gene: its potential as a functional candidate for cardiovascular disease. Clin Sci 124: 65-76. doi: 10.1042/CS20120269

|
|
[133]
|
Meng N, Zhang Y, Ma J, et al. (2015) Association of polymorphisms of angiotensin i converting enzyme 2 with retinopathy in type 2 diabetes mellitus among Chinese individuals. Eye 29: 266-271. doi: 10.1038/eye.2014.254

|
|
[134]
|
Fröjdö S, Sjölind L, Parkkonen M, et al. (2005) Polymorphisms in the gene encoding angiotensin I converting enzyme 2 and diabetic nephropathy. Diabetologia 48: 2278-2281. doi: 10.1007/s00125-005-1955-4

|
|
[135]
|
Currie D, McKnight AJ, Patterson CC, et al. (2010) Investigation of ACE, ACE2 and AGTR1 genes for association with nephropathy in type 1 diabetes mellitus. Diabet Med 27: 1188-1194. doi: 10.1111/j.1464-5491.2010.03097.x

|
|
[136]
|
Chaoxin J, Daili S, Yanxin H, et al. (2013) The influence of angiotensin-converting enzyme 2 gene polymorphisms on type 2 diabetes mellitus and coronary heart disease. Eur Rev Med Pharmacol Sci 17: 2654-2659.
|
|
[137]
|
Patel SK, Wai B, Ord M, et al. (2012) Association of ACE2 genetic variants with blood pressure, left ventricular mass, and cardiac function in caucasians with type 2 diabetes. Am J Hypertens 25: 216-222. doi: 10.1038/ajh.2011.188

|
|
[138]
|
Sayed S (2021) COVID-19 and diabetes; Possible role of polymorphism and rise of telemedicine. Prim Care Diabetes 15: 4-9. doi: 10.1016/j.pcd.2020.08.018

|
|
[139]
|
Calcagnile M, Forgez P, Iannelli A, et al. (2021) Molecular docking simulation reveals ACE2 polymorphisms that may increase the affinity of ACE2 with the SARS-CoV-2 spike protein. Biochimie 180: 143-148. doi: 10.1016/j.biochi.2020.11.004

|
|
[140]
|
Calcagnile M, Forgez P, Iannelli A, et al. (2020) ACE2 polymorphisms and individual susceptibility to SARS-CoV-2 infection: insights from an in silico study. bioRxiv .
|
|
[141]
|
Hamet P, Pausova Z, Attaoua R, et al. (2021) SARS-COV-2 receptor ACE2 gene is associated with hypertension and severity of COVID 19: interaction with sex, obesity and smoking. Am J Hypertens 34: 367-376. doi: 10.1093/ajh/hpaa223

|
|
[142]
|
Choudhary S, Sreenivasulu K, Mitra P, et al. (2020) Role of genetic variants and gene expression in the susceptibility and severity of COVID-19. Ann Lab Med 41: 129-138. doi: 10.3343/alm.2021.41.2.129

|
|
[143]
|
Paniri A, Hosseini MM, Moballegh-Eslam M, et al. (2021) Comprehensive in silico identification of impacts of ACE2 SNPs on COVID-19 susceptibility in different populations. Gene Rep 22: 100979. doi: 10.1016/j.genrep.2020.100979

|
|
[144]
|
Wee AKH (2021) COVID-19's toll on the elderly and those with diabetes mellitus – Is vitamin B12 deficiency an accomplice? Med Hypotheses 146: 110374. doi: 10.1016/j.mehy.2020.110374

|
|
[145]
|
Gonçalves SEAB, Gonçalves TJM, Guarnieri A, et al. (2021) Association between thiamine deficiency and hyperlactatemia among critically ill patients with diabetes infected by SARS-CoV-2. J Diabetes 13: 413-419. doi: 10.1111/1753-0407.13156

|
|
[146]
|
Rhodes JM, Subramanian S, Laird E, et al. (2021) Perspective: vitamin D deficiency and COVID-19 severity – plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J Intern Med 289: 97-115. doi: 10.1111/joim.13149

|
|
[147]
|
Tort AR, Montelongo EAM, Cuazitl AM, et al. (2020) La deficiencia de vitamina D es un factor de riesgo de mortalidad en pacientes con COVID-19. Rev Sanid Milit 74: 106-113. doi: 10.35366/93773

|
|
[148]
|
Biesalski HK (2020) Vitamin D deficiency and co-morbidities in COVID-19 patients – A fatal relationship? NFS J 20: 10. doi: 10.1016/j.nfs.2020.06.001

|
|
[149]
|
Berridge MJ (2017) Vitamin D deficiency and diabetes. Biochem J 474: 1321-1332. doi: 10.1042/BCJ20170042

|
|
[150]
|
Luo BA, Gao F, Qin LL (2017) The association between vitamin D deficiency and diabetic retinopathy in type 2 diabetes: A meta-analysis of observational studies. Nutrients 9: 307. doi: 10.3390/nu9030307

|
|
[151]
|
Singh SK, Jain R, Singh S (2020) Vitamin D deficiency in patients with diabetes and COVID-19 infection. Diabetes Metab Syndr 14: 1033-1035. doi: 10.1016/j.dsx.2020.06.071

|
|
[152]
|
Jin S, Hu W (2021) Severity of COVID-19 and treatment strategy for patient with diabetes. Front Endocrinol (Lausanne) 12: 602735. doi: 10.3389/fendo.2021.602735

|
|
[153]
|
Ceriello A, Prattichizzo F (2021) Pharmacological management of COVID-19 in type 2 diabetes. J Diabetes Complications 35: 107927. doi: 10.1016/j.jdiacomp.2021.107927

|
|
[154]
|
Deng F, Gao D, Ma X, et al. (2021) Corticosteroids in diabetes patients infected with COVID-19. Ir J Med Sci 190: 29-31. doi: 10.1007/s11845-020-02287-3

|
|
[155]
|
Stoian AP, Catrinoiu D, Rizzo M, et al. (2021) Hydroxychloroquine, COVID-19 and diabetes. Why it is a different story. Diabetes Metab Res Rev 37: e3379. doi: 10.1002/dmrr.3379

|
|
[156]
|
Khunti K, Knighton P, Zaccardi F, et al. (2021) Prescription of glucose-lowering therapies and risk of COVID-19 mortality in people with type 2 diabetes : a nationwide observational study in England. Lancet Diabetes Endocrinol 9: 293-303. doi: 10.1016/S2213-8587(21)00050-4

|
|
[157]
|
Gregory JM, Slaughter JC, Duffus SH, et al. (2021) COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic's impact in type 1 and type 2 diabetes. Diabetes Care 44: 526-532. doi: 10.2337/dc20-2260

|
|
[158]
|
Fisher L, Polonsky W, Asuni A, et al. (2020) The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: a national cohort study. J Diabetes Complications 34: 107748. doi: 10.1016/j.jdiacomp.2020.107748

|
|
[159]
|
Ruissen MM, Regeer H, Landstra CP, et al. (2021) Increased stress, weight gain and less exercise in relation to glycemic control in people with type 1 and type 2 diabetes during the COVID-19 pandemic. BMJ Open Diabetes Res Care 9: e002035. doi: 10.1136/bmjdrc-2020-002035

|
|
[160]
|
Holman N, Knighton P, Kar P, et al. (2020) Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol 8: 823-833. doi: 10.1016/S2213-8587(20)30271-0

|
|
[161]
|
Beliard K, Ebekozien O, Demeterco-Berggren C, et al. (2021) Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: data from a multi-site surveillance registry. J Diabetes 13: 270-272. doi: 10.1111/1753-0407.13141

|
|
[162]
|
Vamvini M, Lioutas VA, Middelbeek RJW (2020) Characteristics and diabetes control in adults with type 1 diabetes admitted with covid-19 infection. Diabetes Care 43: e120-e122. doi: 10.2337/dc20-1540

|
|
[163]
|
Al Hayek AA, Robert AA, Alotaibi ZK, et al. (2020) Clinical characteristics of hospitalized and home isolated COVID-19 patients with type 1 diabetes. Diabetes Metab Syndr 14: 1841-1845. doi: 10.1016/j.dsx.2020.09.013

|
|
[164]
|
Unsworth R, Wallace S, Oliver NS, et al. (2020) New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K.. Diabetes Care 43: e170-e171. doi: 10.2337/dc20-1551

|
|
[165]
|
Suwanwongse K, Shabarek N (2021) Newly diagnosed diabetes mellitus, DKA, and COVID-19: causality or coincidence? A report of three cases. J Med Virol 93: 1150-1153. doi: 10.1002/jmv.26339

|
|
[166]
|
Dimeglio LA, Albanese-O'neill A, Muñoz CE, et al. (2020) COVID-19 and children with diabetesdupdates, unknowns, and next steps: first, do no extrapolation. Diabetes Care 43: 2631-2634. doi: 10.2337/dci20-0044

|
|
[167]
|
Cameron FJ, Scratch SE, Nadebaum C, et al. (2014) Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care 37: 1554-1562. doi: 10.2337/dc13-1904

|
|
[168]
|
Kamrath C, Mönkemöller K, Biester T, et al. (2020) Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. JAMA 324: 801-804. doi: 10.1001/jama.2020.13445

|
|
[169]
|
Rabbone I, Schiaffini R, Cherubini V, et al. (2020) Has COVID-19 delayed the diagnosis and worsened the presentation of type 1 diabetes in children? Diabetes Care 43: 2870-2872. doi: 10.2337/dc20-1321

|
|
[170]
|
Powers AC, Aronoff DM, Eckel RH (2021) COVID-19 vaccine prioritisation for type 1 and type 2 diabetes. Lancet Diabetes Endocrinol 9: 140-141. doi: 10.1016/S2213-8587(21)00017-6

|
|
[171]
|
Boddu SK, Aurangabadkar G, Kuchay MS (2020) New onset diabetes, type 1 diabetes and COVID-19. Diabetes Metab Syndr 14: 2211-2217. doi: 10.1016/j.dsx.2020.11.012

|
|
[172]
|
Kuchay MS, Reddy PK, Gagneja S, et al. (2020) Short term follow-up of patients presenting with acute onset diabetes and diabetic ketoacidosis during an episode of COVID-19. Diabetes Metab Syndr 14: 2039-2041. doi: 10.1016/j.dsx.2020.10.015

|
|
[173]
|
Marchand L, Pecquet M, Luyton C (2020) Type 1 diabetes onset triggered by COVID-19. Acta Diabetol 57: 1265-1266. doi: 10.1007/s00592-020-01570-0

|
|
[174]
|
Caruso P, Longo M, Esposito K, et al. (2020) Type 1 diabetes triggered by covid-19 pandemic: a potential outbreak? Diabetes Res Clin Pract 164: 108219. doi: 10.1016/j.diabres.2020.108219

|
|
[175]
|
Goyal H, Sachdeva S, Perisetti A, et al. (2021) Hyperlipasemia and potential pancreatic injury patterns in COVID-19: a marker of severity or innocent bystander? Gastroenterology 160: 946-948.e2. doi: 10.1053/j.gastro.2020.10.037

|
|
[176]
|
Patnaik RNK, Gogia A, Kakar A (2020) Acute pancreatic injury induced by COVID-19. IDCases 22: e00959. doi: 10.1016/j.idcr.2020.e00959

|
|
[177]
|
Bruno G, Fabrizio C, Santoro CR, et al. (2021) Pancreatic injury in the course of coronavirus disease 2019: a not-so-rare occurrence. J Med Virol 93: 74-75. doi: 10.1002/jmv.26134

|
|
[178]
|
Ghosh A, Gupta V, Misra A (2020) COVID19 induced acute pancreatitis and pancreatic necrosis in a patient with type 2 diabetes. Diabetes Metab Syndr 14: 2097-2098. doi: 10.1016/j.dsx.2020.10.008

|
|
[179]
|
Alharmi RAR, Fateel T, Sayed Adnan J, et al. (2021) Acute pancreatitis in a patient with COVID-19. BMJ Case Rep 14: e239656. doi: 10.1136/bcr-2020-239656

|
|
[180]
|
Wang F, Wang H, Fan J, et al. (2020) Pancreatic injury patterns in patients with coronavirus disease 19 pneumonia. Gastroenterology 159: 367-370. doi: 10.1053/j.gastro.2020.03.055

|
|
[181]
|
Pribadi RR, Simadibrata M (2021) Increased serum amylase and/or lipase in coronavirus disease 2019 (COVID-19) patients: is it really pancreatic injury? JGH Open 5: 190-192. doi: 10.1002/jgh3.12436

|
|
[182]
|
Coate KC, Cha J, Shrestha S, et al. (2020) SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in β cells. Cell Metab 32: 1028-1040.e4. doi: 10.1016/j.cmet.2020.11.006

|
|
[183]
|
Kusmartseva I, Wu W, Syed F, et al. (2020) Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab 32: 1041-1051.e6. doi: 10.1016/j.cmet.2020.11.005

|
|
[184]
|
Müller JA, Groß R, Conzelmann C, et al. (2021) SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab 3: 149-165. doi: 10.1038/s42255-021-00347-1

|









 DownLoad:
DownLoad: