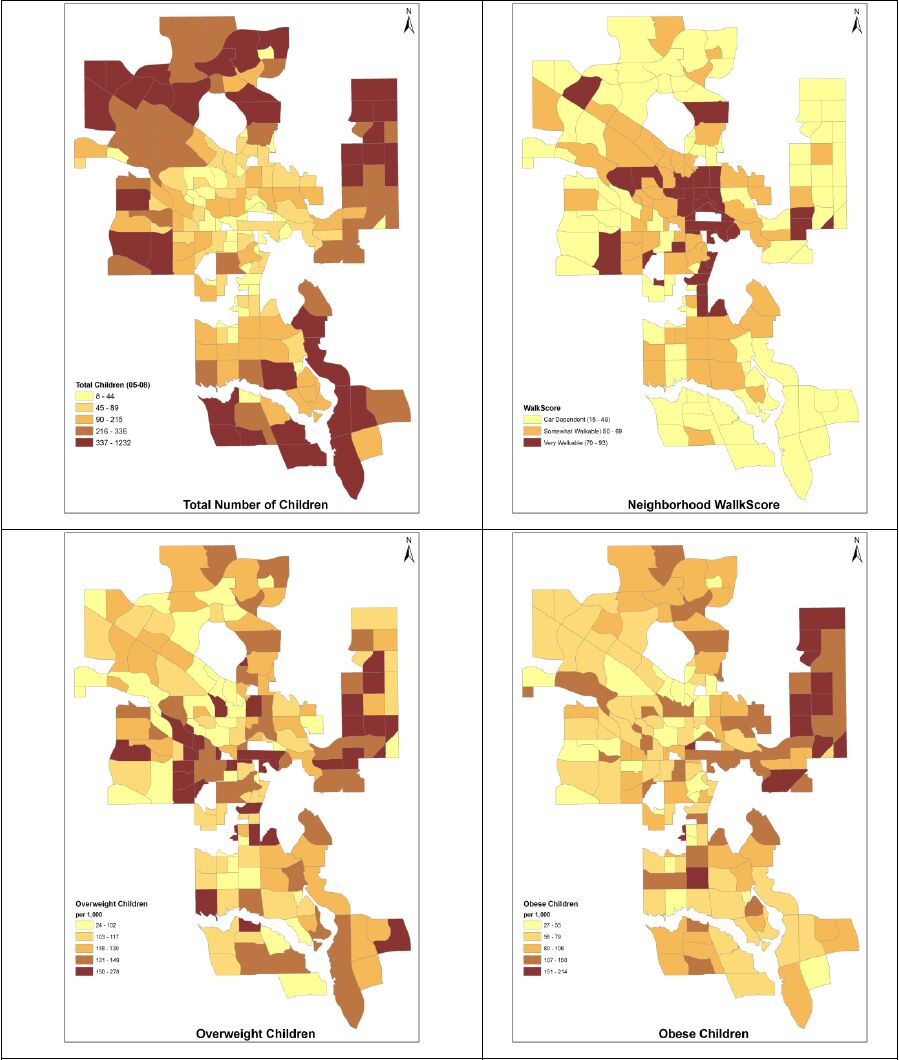
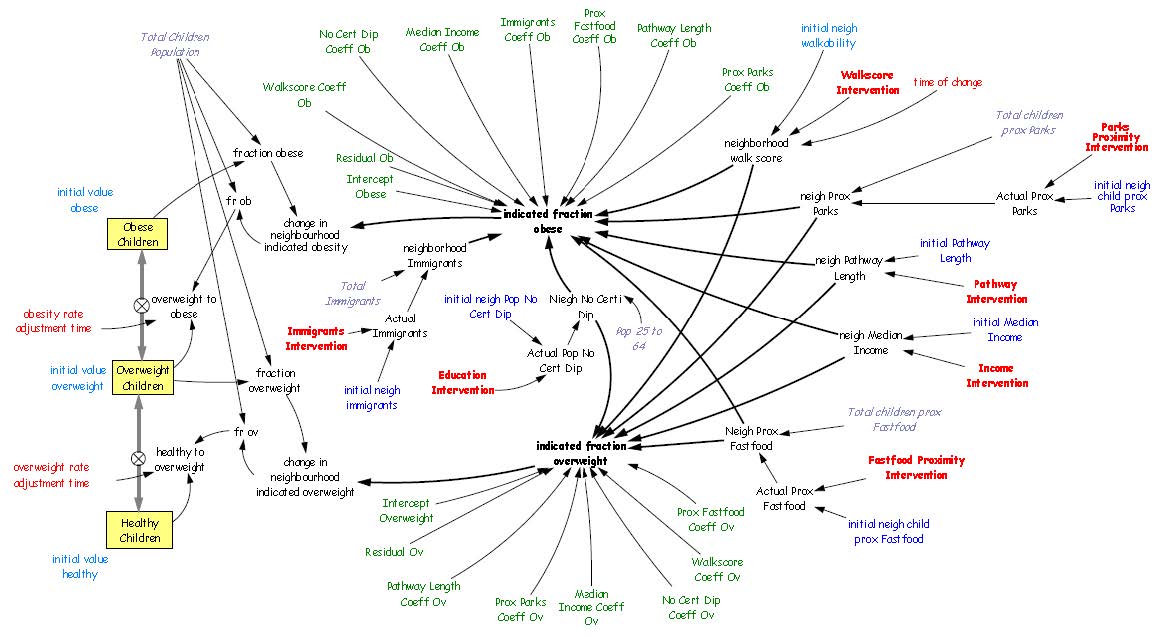
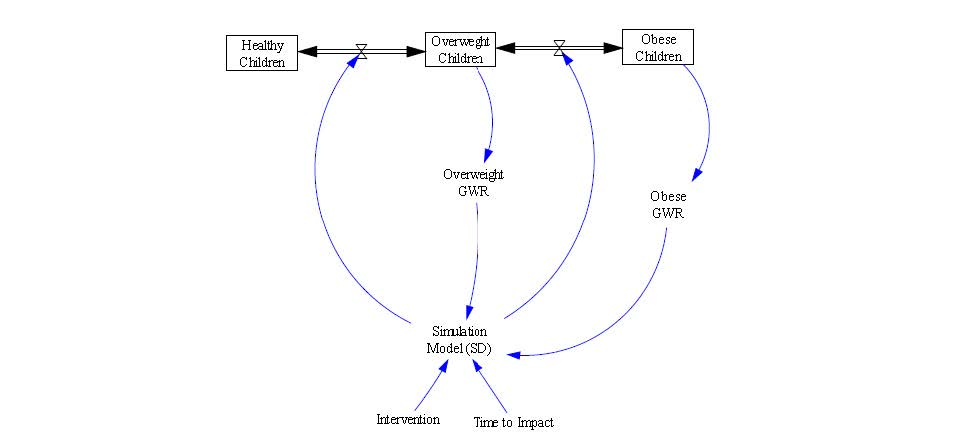
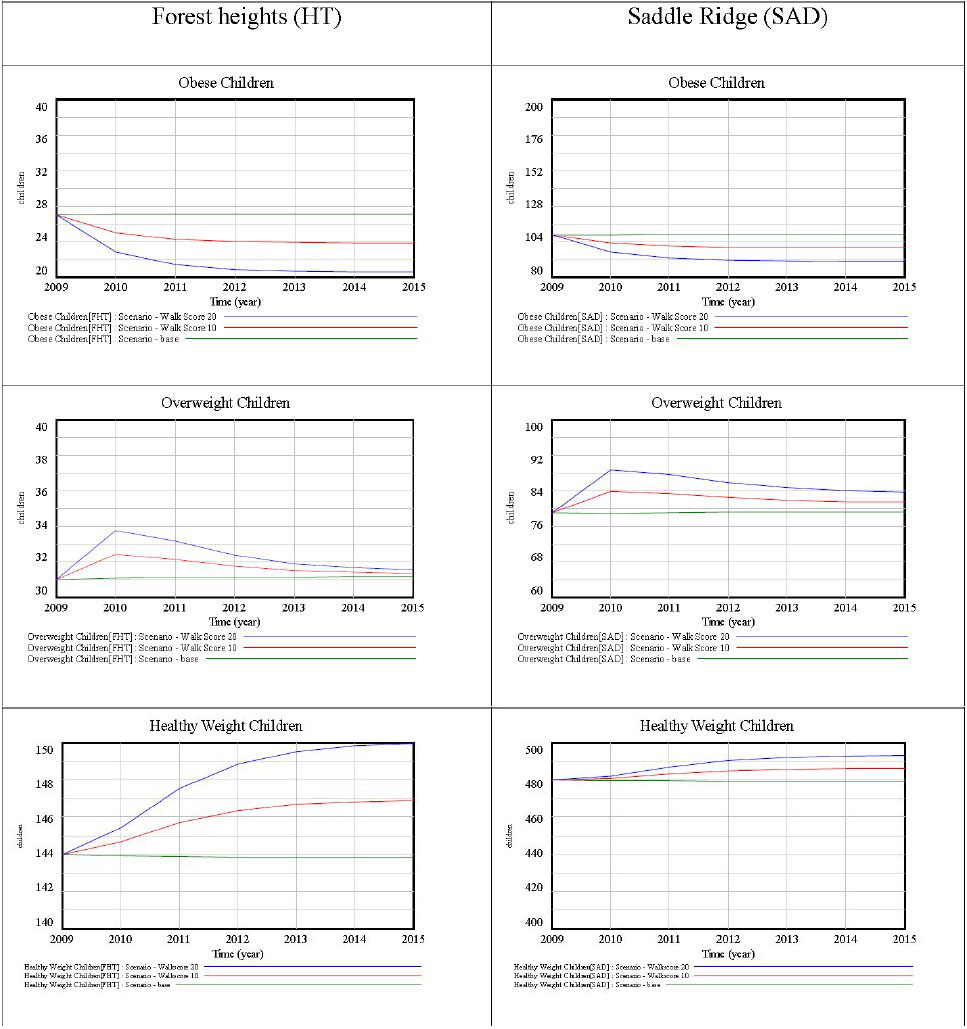
Body weight is an important indicator of current and future health and it is even more critical in children, who are tomorrow’s adults. This paper analyzes the relationship between childhood obesity and neighbourhood walkability in Calgary, Canada. A multivariate analytical framework recognizes that childhood obesity is also associated with many factors, including socioeconomic status, foodscapes, and environmental factors, as well as less measurable factors, such as individual preferences, that could not be included in this analysis. In contrast with more conventional global analysis, this research employs localized analysis and assesses need-based interventions. The one-size-fit-all strategy may not effectively control obesity rates, since each neighbourhood has unique characteristics that need to be addressed individually. This paper presents an innovative framework combining local analysis with simulation modeling to analyze childhood obesity. Spatial models generally do not deal with simulation over time, making it cumbersome for health planners and policy makers to effectively design and implement interventions and to quantify their impact over time. This research fills this gap by integrating geographically weighted regression (GWR), which identifies vulnerable neighbourhoods and critical factors for childhood obesity, with simulation modeling, which evaluates the impact of the suggested interventions on the targeted neighbourhoods. Neighbourhood walkability was chosen as a potential target for localized interventions, owing to the crucial role of walking in developing a healthy lifestyle, as well as because increasing walkability is relatively more feasible and less expensive then modifying other factors, such as income. Simulation results suggest that local walkability interventions can achieve measurable declines in childhood obesity rates. The results are encouraging, as improvements are likely to compound over time. The results demonstrate that the integration of GWR and simulation modeling is effective, and the proposed framework can assist in designing local interventions to control and prevent childhood obesity.
1.
Introduction
Consumption of fresh vegetables has increased worldwide as a result of their nutritional value and the growing interest of consumers for a healthy diet [1]. There is a variety of these produce, including conventional (non-organic) and organic fresh vegetables. Organic farming implies a very limited use of chemical fertilizers and synthetic pesticides, as well as the absence of routine administration of growth hormones and antibiotics to livestock [2]. For these reasons, consumption of organic fresh produce is continuously gaining preference among consumers during last decades, and consequently organic farming has been also proportionally increased. Besides, to facilitate consumption of fresh produce, a variety of them is marketed in a ready-to-eat form that does not require any additional sanitizing process prior to consumption (fresh-cut vegetables) [3,4].
During last decades the foodborne outbreaks due to consumption of fresh produce has also increased, most of them associated to bacterial (mainly to species belonging to the Enterobacteriaceae family) contamination [5,6,7]. Fresh vegetables contain non-pathogenic epiphytic bacteria, and can be contaminated with pathogenic and non-pathogenic bacteria from human and animal sources at any step from farm (growth and harvest) to the consumers [8,9,10,11]. An important source of contamination is the use of manure and irrigation water that can incorporate bacteria from animal and/or human origin to soil and vegetables [12,13,14,15]. We have previously described that fresh vegetables marketed in Valencia (Spain) harbor at high frequency non-pathogenic Enterobacteriaceae species belonging to genera Enterobacter (mainly E. cloacae) and Klebsiella, and that Enterobacteriaceae species are also found in samples of organic produce [16,17,18].
A significant concern derived from the presence of enterobacteria in fresh vegetables is related to the antibiotic resistances, as Enterobacteriaceae isolates from fresh produce are often resistant to antibiotics [19,20,21]. Most enterobacteria isolates from fresh vegetables from Valencia are resistant to penicillins (amoxillin/clavulanic and ampicillin) and in a minor extent to other antibiotics [16,17]. Antibiotic resistant bacteria in fresh vegetables can arise from the use of antibiotics in husbandry (through manure and contaminated irrigation water) and plant agriculture, which either can incorporate resistant bacteria into vegetables or select resistance in vegetable microbiota [12,13,14,15]. In addition, the fact that fresh vegetables are often eaten raw points another issue: ingestion of antibiotic resistant enterobacteria by the consumer may result in the transmission of resistances to other commensal or pathogenic gut microbiota through horizontal mechanisms of gene transfer [20,21,22,23]. It should be noted that enterobacteria from fresh produce may, at least transiently, colonize the gastrointestinal tract, a niche considered as a hot spot for gene transfer among bacteria [24]. We have previously qualified this process as a silent food safety concern [16], which may contribute to disseminate antibiotic resistances within the community and to agammaavate the increasing problem of the antibiotic resistance in Gram-negative bacteria both in hospital settings and in the community [25,26].
Therefore, it seems relevant to compare the presence of enterobacteria in different fresh vegetable produce (conventional, organic, and fresh-cut and prepared salads), their identification at the species level and the determination of their antibiotic resistances, in order to find out any differences among types of samples related with their hygienic quality and safety for consumers; so in this work we have extended our former studies to a higher number of bacterial species and fresh vegetable types (above cited) and compiled all data in order to show a comparison among them.
2.
Materials and methods
2.1. Sample collections
Samples (n: 230) (carrot, ruccola/arugula and various lettuce and tomato types) of conventional vegetables (n: 90) and fresh-cut vegetables (conventionally grown) (n: 60) were purchased from supermarkets and retail greengrocers in the city of Valencia, and organic produce (n: 50) were purchased in retail greengrocers specialized in produce grown in the area of Valencia. All samples were purchased in bulk, except fresh-cut vegetables that were purchased in unit packets. Prepared salads (n: 30) were obtained from the daily menu of primary schools and restaurants in the city of Valencia.
2.2. Determination of coliform burden and species identification
All microbiological procedures were performed according to standard methods as described elsewhere [16,17,18]. Briefly, selected portions of each sample (2–5 g) were homogenized in a Classic Masticator for 1 min at room temperature. In the case of salads, parts all ingredients were taken to obtain the homogenates. Serial dilutions (10-1, 10-2 and 10-3) were used to determine the most probably number of coliform bacteria/g (MPN) by growth and gas formation following 24–48 h incubation in Brilliant Green Bile Lactose Broth (BGBL) (Conda, Spain); the detection limits of the method range between < 3 and > 2400 coliforms/g. Single colonies were isolated by plating on selective EMB Levine agar (Liofilchem, Italy) and incubation for 24 h at 37 ℃. Identification of isolates at the species level was performed by biochemical test using the BBL Crystal E/NF identification system (Becton Dickinson, USA) according to the instructions and software provided by the manufacturer. In order to quantify the diversity of bacterial species isolated from each vegetable type, we calculated the diversity ratio (DR), defined as ratio between the number of the different species identified and the total number of isolates (the maximum theoretical value of DR is one, which indicates that each isolate belongs to a different species).
2.3. Antibiotic susceptibility test
Determination of antibiotic susceptibility was performed by disk diffusion-based tests according to standard microbiological procedures previously described [16,17,18]. Eleven chemotherapeutic agents (most of them antibiotics) commonly used against Gram-negative bacteria were tested: amoxicillin/clavulanic, ampicillin, cefotaxime, ceftazidime, ciprofloxacin, chloramphenicol, cotrimoxazole, streptomycin, gentamicin, nitrofurantoin, and tetracycline (Liofilchem, Italy). Two indexes have been used to show the antibiotic resistance level of each bacterial species: the resistance index 11 (RI11) that indicates the average absolute number of resistances to the eleven antibacterial agents tested (with values ranging between 0 and 11), and the relative resistance index (RRI) defined as the ratio RI11/11 (with values ranging between 0 and 1).
3.
Results and discussion
3.1. Coliform burden and bacterial species identified in fresh vegetables
Results of a total of 200 vegetable samples were compared in this study, as well as 30 prepared salads. The coliform burden of all vegetable samples was determined as an indicator of their hygienic quality, as coliforms include Enterobacteriaceae species (mainly belonging to the genera Enterobacter, Klebsiella,
Escherichia and Serratia) widely distributed in nature, including vegetables epiphytic bacteria and enterobacteria of the gastrointestinal tract of mammals. Results (Table 1) showed that fresh vegetables were the most contaminated, compared to conventional and organic produce, looking at both negative results and samples with high coliform burden. Prepared salads showed the highest levels of coliform burden, result that is not surprising as the analyzed samples of salads contained various vegetables ingredients and their preparation requires greater manipulation.
The identified bacterial species in each type of fresh produce are shown in Tables 2 and 3. The methodology used for bacterial isolation allowed the identification of coliforms as well as other non- coliform Enterobacteriaceae and other Gram-negative species that are able to growth in BGBL cultures. Coliform isolates were the most frequently identified in all samples (67–85%), except in organic fresh vegetables, where most frequent isolates corresponded to non-coliform Enterobacteriaceae species (62%). A low proportion of non-Enterobacteriaceae Gram-negative isolates were only found in conventional, and particularly in fresh-cut vegetables. The average number of isolates per sample was similar in conventional and organic vegetables (0.69 and 0.74, respectively), and higher in fresh-cut and prepared salads (1.05 and 1.10, respectively); these results suggest that a greater manipulation of the produce represents a greater risk of contamination and therefore is associated with a higher number of isolates (Table 2).
The species most frequently isolated from conventional, fresh-cut vegetables and salads was Enterobacter cloacae, whereas in organic produce it was Pantoea agglomerans, a non-coliform Enterobacteriaceae species; the second most frequent species were Klebsiella oxytoca in conventional vegetables and salads, Serratia marcescens and E. cloacae in organic samples, and Klebsiella pneumoniae, Cronobacter sakazakii and Flavimonas orizyhabitans in fresh-cut produce (Table 3). Looking at the diversity of the isolates, the lower diversity ratio was observed in conventional fresh vegetables (DR: 0.16), followed by fresh-cut vegetables (DR: 0.24), and organic produce (DR: 0.35), whereas salads had an intermediate value (DR: 0.27).
When grouped by genera, Enterobacter and Klebsiella spp. represented roughly 60–77% of isolates from conventional, fresh-cut vegetables and prepared salads (about 50% corresponding to Enterobacter spp.), although the percentage of Klebsiella spp. was lower in fresh-cut vegetables (8%) than in samples of conventional and prepared salads (roughly 30%). In organic produce Enterobacter spp. represented 27% of isolates, whereas only 8% belonged to Klebsiella spp. Other genera, as Pantoea and Serratia represented 40% of isolates in organic produce (Table 4). Isolates belonging to non-Enterobacteriaceae species such as Flavimonas (F. orizyhabitans) and Pseudomonas (Ps. putida) were only found in fresh-cut samples.
Taken together these results indicate that organic vegetables contain a different pattern of bacterial burden, as compared with conventional fresh produce. These differences may arise from the distinct farming practices, as both type of samples are mostly grown in the same geographical area. Furthermore, diversity of bacterial burden in organic produce was the highest among all sample types, probably due to the limited use of chemical fertilizers and synthetic pesticides/antibiotics in organic farming practice [2,27], which may allow the growth of non-resistant bacteria, whereas the use of pesticides and antibiotics in conventional farming may reduce bacterial diversity. Besides, fresh-cut vegetables showed bacterial burdens and bacterial diversity higher than conventional fresh produce, despite to be ready for consumption. Bacteria possess mechanisms for adhesion to vegetable surfaces, which make inefficient the washing/sanitizing methods to remove/inactivate bacteria, and manipulation of vegetables to obtain fresh-cut produce is an additional risk of contamination [4,6,8,10,28]; besides when they are cut, bacteria can enter into vegetable damaged tissues and access to nutrient molecules that favor bacterial proliferation and limit the efficacy of conventional sanitizing methods [3,4,29,30]. Results of prepared salads indicate that vegetable ingredients maintain a significant bacterial burden that is ingested by the consumer, showing the inefficiency of tap water washing methods for bacterial removal at home [31], and their bacterial content and diversity suggest that the salads analyzed in this study were prepared probably using mostly conventional produce. Furthermore, most of the identified isolates belonged to opportunist pathogenic species, and therefore their presence in fresh vegetables may be regarded as a potential risk for the immunocompromised or debilitated consumers, and a source for cross-contaminating other foods at home. In summary, our results, together with other reports, support the question raised by Vestrheim et al. [32]: “are ready-to-eat salads ready to eat?” and point to the need of better methods to diminish the bacterial content of these produce [4,9,10,30].
3.2. Resistance to chemotherapeutic agents in bacterial isolates from fresh vegetables
Next, resistance to eleven chemotherapeutic agents was determined in all isolates. Results of resistances in isolates from each vegetable type are shown in Table 5. Only a low percentage of isolates showed no resistances in all cases (9.5–13.5%). Most isolates from each vegetable type were resistant to ampicillin (64–85 %) and amoxicillin/clavulanic (70–77%, except in the case of organic produce, were about 40% of isolates were resistant). Resistance to cephalosporins was detected at very low frequency (0–3%), except for resistance to cefotaxime in isolates from organic produce (11%). Resistance to nitrofurantoin was relatively frequent, and higher in isolates from organic and fresh-cut vegetables (16% and 19% of resistant isolates, respectively) than in conventional and prepared salads (8% and 6%, respectively). Resistance to tetracycline was also found, particularly in conventional produce (11% of resistant isolates) as compared to other vegetable types (roughly 3–5% of resistance). Resistance to other chemotherapeutic agents was also very low in most cases (roughly between 0–3%), particularly to ciprofloxacin (0% for all vegetable types). The differences in the resistance patterns between isolates from conventional and organic produce may be caused, at least partially, by the differences in the bacterial species isolated from both produce, above described. Remarkably, all isolates from organic vegetable showed no resistances to 5 out the 11 antibacterial agents tested, whereas isolates from conventional produce showed no resistances only to two agents. These results suggest that organic farming practices may have a relative but significant effect in avoiding selection of resistant bacteria. The resistence pattern of isolates from fresh-cut vegetables and salads resembles that of conventional produce, with some exceptions above cited.
Multi-resistances to more than one chemotherapeutic agents in isolates from each type of fresh produce are shown in Table 6. Comparison among vegetable types does not reveal evident differences. Almost 80% of isolates from conventional produce were resistant to more than one chemotherapeutic agent, a value higher than in other vegetable types: fresh-cut produce (54%), salads (52%) and particularly organic produce (43%). Resistance to two agents was the most frequently observed in isolates from all vegetable types (almost 60% in conventional produce), except for organic produce, where most isolates showed only one resistance (43%). Percentages of multi-resistant isolates to 3–4 agents were similar in all produce types (about 16%), except in prepared salads (6%), and only two isolates were resistant to five antibacterial agents (one from conventional vegetables and another one from fresh-cut produce). Comparison of the RI11 and RRI values indicates a higher levels of resistance in isolates from conventional produce compared to organic vegetables; fresh-cut vegetables showed intermediate values between conventional and organic vegetables, and isolates from salads had the lower values. These results again suggest a higher level of resistance in isolates from conventional produce, supporting the notion that organic farming may contribute to diminish selection and spreading of resistant bacteria. Results concerning resistant isolates from salads were intermediate between conventional, organic and fresh-cut produce, and the absence of multi-resistant isolates to 4–5 agents (probably due to the lower number of samples/isolates analyzed) accounts for lower RI11 and RRI values.
Multi-resistances to chemotherapeutic agents in bacterial species isolated from fresh vegetables, grouped by resistance patterns, are shown in Table 7. The 13 multi-resistant isolates to 4-5 agents belonged to E. cloacae (5 isolates), S. marcescens (2 isolates), K. pneumoniae, C. freundii, Cr. sakazakii, P. agglomerans and A. baumanii (one isolate each) species, including isolates from conventional, fresh-cut and organic vegetables (see Table 6).
The significance of the resistance indexes should be considered only for species with the higher amount of isolates; comparison of RI11 and RRI among species with more than 10 isolates indicates that the highest values of RRI corresponded to E. cloacae (0.192), and K. pneumoniae (0.173), followed by S. marcescens and K. oxytoca (0.136 and 0.134, respectively), and the lowest value was found for P. agglomerans isolates (0.095).
The presence of antibiotic-resistant bacteria in produce that are often eaten raw points out to the role of their consumption as a means for the entry of resistant bacteria into the consumer [20,21,22]. In addition, bacteria associated with fresh produce have a high diversity of self-transmissible multiple resistance plasmids that could be transferred to gut bacteria (both non-pathogen and pathogen species) through horizontal gene transfer mechanisms [23,24], therefore contributing to the spreading of resistant bacteria in the community, and consequently to agammaavate the increasingly growing problem of infections caused by resistant Gram-negative bacteria [25,26].
4.
Conclusions
The presence of Enterobacteriaceae (and other Gram-negative) species in all type of fresh vegetables (including fresh-cut vegetables and prepared salads) confirms that their consumption may be considered (i) as a food safety concern, even in the absence of pathogenic species, as most of the identified isolates correspond to opportunistic pathogens able to cause infection in immunocompromised individuals, and (ii) as a means for the of entry resistant bacteria into the consumer, therefore contributing to the spreading of resistant bacteria into the community. Thus, it could be relevant to include the detection of antibiotic-resistant non-pathogenic Enterobacteriaceae species in fresh vegetables in the epidemiological surveillance routine in order obtain valuable information about the extent of dissemination of antibiotic-resistant bacteria into the non-hospital environment, and to evaluate the potential role of consumption of fresh vegetables in spreading resistances into community. Our results indicate that sanitizing methods for fresh vegetables are not efficient enough to diminish their microbial burden, and therefore improved sanitizing processes are needed. Consequently, the development of farming strategies to prevent microbial contamination of fresh vegetables is an important key to increase the safety of their consumption. Finally, organic farming practices may favor bacterial diversity and a minor level of antibiotic-resistant bacteria in fresh produce, although a minor content of antibiotic-resistant bacteria would be desirable as an indicator of the quality of organic fresh produce.
Acknowledgments
This work was supported by the Research Funds of the University of Valencia (Spain).
Conflict of interest
The authors declare no conflict of interest.









 DownLoad:
DownLoad: