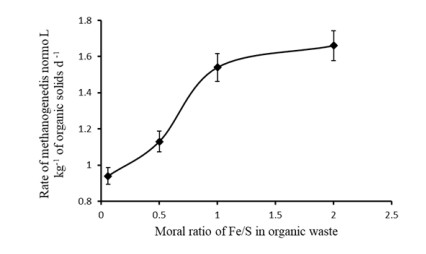
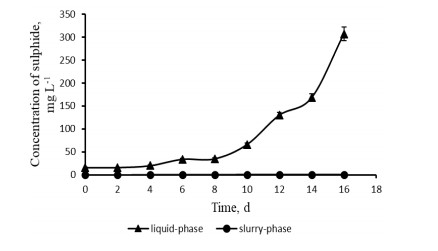
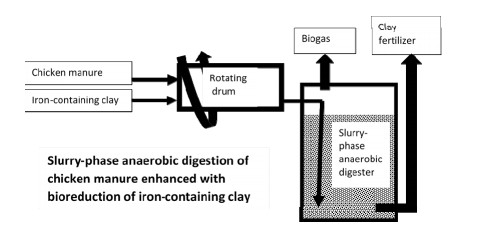
It is shown in this review that addition of clay minerals and hematite iron ore can significantly enhance anaerobic digestion of chicken manure. Liquid-phase anaerobic digestion of chicken manure consumes a lot of fresh water and energy to keep waste as a suspension. Meanwhile, anaerobic digestion of chicken manure in clay slurry without stirring could minimize energy and water consumption because the initial acceptable content of organic solids can be increased. For example, this content can be increased from 5% (w v-1) in suspension of chicken manure for liquidphase anaerobic digestion up to 15% (w v-1) in the slurry of chicken manure for slurry-phase anaerobic digestion than can save up to 13.3 L of water per kilogram of dry organic solids. The slurry-phase anaerobic digestion of nitrogen-, sulphur-, and fat-containing organic wastes can be enhanced using microbial reduction of Fe(Ⅲ) in clay or in hematite iron ore. This is due to adsorption or precipitation of such inhibitors of microbial acidogenesis and methanogenesis as ammonium, sulphide, long-chain fatty acids, humic and fulvic acids with clay or ferrous ions. For example, maximum concentration of ammonium decreased from 11.4 g L-1 during liquid-phase anaerobic digestion to 1.4 g L-1 during slurry-phase process due to adsorption of ammonium ions on clay. Addition of iron-containing clay to slurry-phase anaerobic reactor removed dissolved sulphide totally due to its precipitation with ferrous ions that are produced by bioreduction of Fe(Ⅲ) in clay. Slurry-phase anaerobic digestion enhanced with bioreduction of Fe(Ⅲ) minerals is also more effective process in terms of environmental safety than widely used liquid-phase anaerobic digestion because of an absence of water supply and wastewater effluent.
1.
Introduction
Baló's concentric sclerosis (BCS) was first described by Marburg [1] in 1906, but became more widely known until 1928 when the Hungarian neuropathologist Josef Baló published a report of a 23-year-old student with right hemiparesis, aphasia, and papilledema, who at autopsy had several lesions of the cerebral white matter, with an unusual concentric pattern of demyelination [2]. Traditionally, BCS is often regarded as a rare variant of multiple sclerosis (MS). Clinically, BCS is most often characterized by an acute onset with steady progression to major disability and death with months, thus resembling Marburg's acute MS [3,4]. Its pathological hallmarks are oligodendrocyte loss and large demyelinated lesions characterized by the annual ring-like alternating pattern of demyelinating and myelin-preserved regions. In [5], the authors found that tissue preconditioning might explain why Baló lesions develop a concentric pattern. According to the tissue preconditioning theory and the analogies between Baló's sclerosis and the Liesegang periodic precipitation phenomenon, Khonsari and Calvez [6] established the following chemotaxis model
where ˜u, ˜v and ˜w are, respectively, the density of activated macrophages, the concentration of chemoattractants and density of destroyed oligodendrocytes. ˉu and ˉw represent the characteristic densities of macrophages and oligodendrocytes respectively.
By numerical simulation, the authors in [6,7] indicated that model (1.1) only produces heterogeneous concentric demyelination and homogeneous demyelinated plaques as χ value gradually increases. In addition to the chemoattractant produced by destroyed oligodendrocytes, "classically activated'' M1 microglia also can release cytotoxicity [8]. Therefore we introduce a linear production term into the second equation of model (1.1), and establish the following BCS chemotaxis model with linear production term
Before going to details, let us simplify model (1.2) with the following scaling
then model (1.2) takes the form
where Ω⊂Rn(n≥1) is a smooth bounded domain, η is the outward normal vector to ∂Ω, ∂η=∂/∂η, δ balances the speed of the front and the intensity of the macrophages in damaging the myelin. The parameters χ,α and δ are positive constants as well as β,γ are nonnegative constants.
If δ=0, then model (1.3) is a parabolic-elliptic chemotaxis system with volume-filling effect and logistic source. In order to be more line with biologically realistic mechanisms, Hillen and Painter [9,10] considered the finite size of individual cells-"volume-filling'' and derived volume-filling models
q(u) is the probability of the cell finding space at its neighbouring location. It is also called the squeezing probability, which reflects the elastic properties of cells. For the linear choice of q(u)=1−u, global existence of solutions to model (1.4) in any space dimension are investigated in [9]. Wang and Thomas [11] established the global existence of classical solutions and given necessary and sufficient conditions for spatial pattern formation to a generalized volume-filling chemotaxis model. For a chemotaxis system with generalized volume-filling effect and logistic source, the global boundedness and finite time blow-up of solutions are obtained in [12]. Furthermore, the pattern formation of the volume-filling chemotaxis systems with logistic source and both linear diffusion and nonlinear diffusion are shown in [13,14,15] by the weakly nonlinear analysis. For parabolic-elliptic Keller-Segel volume-filling chemotaxis model with linear squeezing probability, asymptotic behavior of solutions is studied both in the whole space Rn [16] and on bounded domains [17]. Moreover, the boundedness and singularity formation in parabolic-elliptic Keller-Segel volume-filling chemotaxis model with nonlinear squeezing probability are discussed in [18,19].
Very recently, we [20] investigated the uniform boundedness and global asymptotic stability for the following chemotaxis model of multiple sclerosis
subject to the homogeneous Neumann boundary conditions.
In this paper, we are first devoted to studying the local existence and uniform boundedness of the unique classical solution to system (1.3) by using Neumann heat semigroup arguments, Banach fixed point theorem, parabolic Schauder estimate and elliptic regularity theory. Then we discuss that exponential asymptotic stability of the positive equilibrium point to system (1.3) by constructing Lyapunov function.
Although, in the pathological mechanism of BCS, the initial data in model (1.3) satisfy 0<u0(x)≤1,w0(x)=0, we mathematically assume that
It is because the condition (1.5) implies u(x,t0)>0 for any t0>0 by the strong maximum principle.
The following theorems give the main results of this paper.
Theorem 1.1. Assume that the initial data (u0(x),w0(x)) satisfy the condition (1.5). Then model (1.3) possesses a unique global solution (u(x,t),v(x,t),w(x,t)) satisfying
and
Moreover, there exist a ν∈(0,1) and M>0 such that
Theorem 1.2. Assume that β≥0,γ≥0,β+γ>0 and
Let (u, v, w) be a positive classical solution of the problem (1.3), (1.5). Then
Furthermore, there exist positive constants \lambda = \lambda(\chi, \alpha, \gamma, \delta, n) and C = C(|\Omega|, \chi, \alpha, \beta, \gamma, \delta) such that
where (u^{\ast}, v^{\ast}, w^{\ast}) = (1, \frac{\beta+\gamma}{\alpha}, 1) is the unique positive equilibrium point of the model (1.3).
The paper is organized as follows. In section 2, we prove the local existence, the boundedness and global existence of a unique classical solution. In section 3, we firstly establish the uniform convergence of the positive global classical solution, then discuss the exponential asymptotic stability of positive equilibrium point in the case of weak chemotactic sensitivity. The paper ends with a brief concluding remarks.
2.
Boundedness and global existence
The aim of this section is to develop the existence and boundedness of a global classical solution by employing Neumann heat semigroup arguments, Banach fixed point theorem, parabolic Schauder estimate and elliptic regularity theory.
Proof of Theorem 1.1 (ⅰ) Existence. For p\in (1, \infty) , let A denote the sectorial operator defined by
\lambda_{1} > 0 denote the first nonzero eigenvalue of -\Delta in \Omega with zero-flux boundary condition. Let A_{1} = -\Delta+\alpha and X^{l} be the domains of fractional powers operator A^{l}, \; l\geq 0 . From the Theorem 1.6.1 in [21], we know that for any p > n and l\in(\frac{n}{2p}, \frac{1}{2}) ,
We introduce the closed subset
in the space X: = C^{0}([0, T];C^{0}(\bar{\Omega})) , where R is a any positive number satisfying
and T > 0 will be specified later. Note F(u) = \frac{u}{1+u} , we consider an auxiliary problem with F(u) replaced by its extension \tilde{F}(u) defined by
Notice that \tilde{F}(u) is a smooth globally Lipshitz function. Given \hat{u}\in S , we define \Psi\hat{u} = u by first writing
and
then letting v solve
and finally taking u to be the solution of the linear parabolic problem
Applying Agmon-Douglas-Nirenberg Theorem [22,23] for the problem (2.3), there exists a constant C such that
for all t\in(0, T) . From a variation-of-constants formula, we define
First we shall show that for T small enough
for any \hat{u}\in S . From the maximum principle, we can give
and
for all t\in(0, T) . We use inequalities (2.1) and (2.4) to estimate
for all t\in(0, T) . This estimate is attributed to T < 1 and the inequality in [24], Lemma 1.3 iv]
From inequalities (2.5), (2.6) and (2.7) we can deduce that \Psi maps S into itself for T small enough.
Next we prove that the map \Psi is a contractive on S . For \hat{u}_{1}, \hat{u}_{2}\in S , we estimate
Fixing T\in(0, 1) small enough such that
It follows from the Banach fixed point theorem that there exists a unique fixed point of \Psi .
(ⅱ) Regularity. Since the above of T depends on \|u_{0}\|_{L^{\infty}(\Omega)} and \|w_{0}\|_{L^{\infty}(\Omega)} only, it is clear that (u, v, w) can be extended up to some maximal T_{\max}\in(0, \infty] . Let Q_{T} = \Omega \times (0, T] for all T\in (0, T_{\max}) . From u\in C^{0}(\bar{Q}_{T}) , we know that w\in C^{0, 1}(\bar{Q}_{T}) by the expression (2.2) and v\in C^{0}([0, T], W_{p}^{2}(\Omega)) by Agmon-Douglas-Nirenberg Theorem [22,23]. From parabolic L^{p} -estimate and the embedding relation W_{p}^{1}(\Omega)\hookrightarrow C^{\nu}(\bar{\Omega}), \; p > n , we can get u\in W^{2, 1}_{p}(Q_{T}) . By applying the following embedding relation
we can derive u(x, t)\in C^{\nu, \nu/2}(\bar{Q}_{T}) with 0 < \nu\leq 2-\frac{n+2}{p} . The conclusion w\in C^{\nu, 1+\nu/2}(\bar{Q}_{T}) can be obtained by substituting u\in C^{\nu, \nu/2}(\bar{Q}_{T}) into the formulation (2.2). The regularity u\in C^{2+\nu, 1+\nu/2}(\bar{Q}_{T}) can be deduced by using further bootstrap argument and the parabolic Schauder estimate. Similarly, we can get v\in C^{0}((0, T), C^{2+\nu}(\bar{\Omega})) by using Agmon-Douglas-Nirenberg Theorem [22,23]. From the regularity of u we have w\in C^{2+\nu, 1+\nu/2}(\bar{Q}_{T}) .
Moreover, the maximal principle entails that 0 < u(x, t)\leq 1 , 0\leq v(x, t)\leq\frac{\beta+\gamma}{\alpha} . It follows from the positivity of u that \tilde{F}(u) = F(u) and because of the uniqueness of solution we infer the existence of the solution to the original problem.
(ⅲ) Uniqueness. Suppose (u_{1}, v_{1}, w_{1}) and (u_{2}, v_{2}, w_{2}) are two deferent solutions of model (1.3) in \Omega\times [0, T] . Let U = u_{1}-u_{2} , V = v_{1}-v_{2} , W = w_{1}-w_{2} for t\in (0, T) . Then
where we have used that |\nabla v_{1}|\leq K results from \nabla v_{1}\in C^{0}([0, T], C^{0}(\bar{\Omega})).
Similarly, by Young inequality and w_{0}\leq w_{1}\leq 1 , we can estimate
and
Finally, adding to the inequalities (2.9)–(2.11) yields
The results U\equiv 0 , W\equiv0 in \Omega\times(0, T) are obtained by Gronwall's lemma. From the inequality (2.10), we have V\equiv 0 . Hence (u_{1}, v_{1}, w_{1}) = (u_{2}, v_{2}, w_{2}) in \Omega\times(0, T) .
(ⅳ) Uniform estimates. We use the Agmon-Douglas-Nirenberg Theorem [22,23] for the second equation of the model (1.3) to get
for all t\geq 1 and C_{2} is independent of t . From the embedded relationship W_{p}^{1}(\Omega)\hookrightarrow C^{0}({\bar{\Omega}}), \; p > n , the parabolic L^{p} -estimate and the estimation (2.12), we have
for all t\geq 1 . The estimate \|u\|_{C^{\nu, \frac{\nu}{2}}(\bar{\Omega}\times [t, t+1])}\leq C_{4} for all t\geq 1 obtained by the embedded relationship (2.8). We can immediately compute \|w\|_{C^{\nu, 1+\frac{\nu}{2}}(\bar{\Omega}\times [t, t+1])}\leq C_{5} for all t\geq 1 according to the regularity of u and the specific expression of w . Further, bootstrapping argument leads to \|v\|_{C^{0}([t, t+1], C^{2+\nu}(\bar{\Omega}))}\leq C_{6} and \|u\|_{C^{2+\nu, 1+\frac{\nu}{2}}(\bar{\Omega}\times [t, t+1])}\leq C_{7} for all t\geq 1 . Thus the uniform estimation (1.7) is proved.
Remark 2.1. Assume the initial data 0 < u_{0}(x)\leq 1 and w_{0}(x) = 0 . Then the BCS model (1.3) has a unique classical solution.
3.
Exponential stability of positive equilibrium point
In this section we investigate the global asymptotic stability of the unique positive equilibrium point (1, \frac{\beta+\gamma}{\alpha}, 1) to model (1.3). To this end, we first introduce following auxiliary problem
By a similar proof of Theorem 1.1, we get that the problem (3.1) has a unique global classical solution (u_{\epsilon}, v_{\epsilon}, w_{\epsilon}) , and there exist a \nu\in(0, 1) and M_{1} > 0 which is independent of \epsilon such that
Then, motivated by some ideas from [25,26], we construct a Lyapunov function to study the uniform convergence of homogeneous steady state for the problem (3.1).
Let us give following lemma which is used in the proof of Lemma 3.2.
Lemma 3.1. Suppose that a nonnegative function f on (1, \infty) is uniformly continuous and \int_{1}^{\infty}f(t)dt < \; \infty . Then f(t)\rightarrow 0 as t\rightarrow \infty.
Lemma 3.2. Assume that the condition (1.8) is satisfied. Then
where v^{\ast} = \frac{\beta+\gamma}{\alpha} .
Proof We construct a positive function
From the problem (3.1) and Young's inequality, we can compute
We multiply the second equations in system (3.1) by v_{\epsilon}-v^{\ast} , integrate by parts over \Omega and use Young's inequality to obtain
and
Substituting inequality (3.5) into inequality (3.4) to get
where C_{8} = \min\left\{1-\frac{\chi^{2}\beta^{2}}{8\alpha}, 1-\frac{\chi^{2}\gamma^{2}}{8\alpha}\right\} > 0.
Let f(t): = \int_{\Omega}(u_{\epsilon}-1)^{2}+(w_{\epsilon}-1)^{2}dx . Then
It follows from the uniform estimation (3.2) and the Arzela-Ascoli theorem that f(t) is uniformly continuous in (1, \infty) . Applying Lemma 3.1, we have
Combining inequality (3.6) and the limit (3.7) to obtain
Proof of Theorem 1.2 As we all known, each bounded sequence in C^{2+\nu, 1+\frac{\nu}{2}}(\bar{\Omega}\times[1, \infty)) is precompact in C^{2, 1}(\bar{\Omega}\times[1, \infty)) . Hence there exists some subsequence \{u_{\epsilon_{n}}\}_{n = 1}^{\infty} satisfying \epsilon_{n}\rightarrow0 as n\rightarrow \infty such that
Similarly, we can get
and
Combining above limiting relations yields that (u_{\ast}, v_{\ast}, w_{\ast}) satisfies model (1.3). The conclusion (u_{\ast}, v_{\ast}, w_{\ast}) = (u, v, w) is directly attributed to the uniqueness of the classical solution of the model (1.3). Furthermore, according to the conclusion, the strong convergence (3.3) and Diagonal line method, we can deduce
By applying Gagliardo-Nirenberg inequality
comparison principle of ODE and the convergence (3.8), the uniform convergence (1.9) is obtained immediately.
Since \lim_{t\rightarrow \infty}\|u(\cdot, t)-1\|_{L^{\infty}(\Omega)} = 0 , so there exists a t_{1} > 0 such that
Using the explicit representation formula of w
and the inequality (3.10), we have
Multiply the first two equations in model (1.3) by u-1 and v-v^{\ast} , respectively, integrate over \Omega and apply Cauchy's inequality, Young's inequality and the inequality (3.10), to find
Combining the estimations (3.11)–(3.13) leads us to the estimate
Let y(t) = \int_{\Omega}(u-1)^{2}dx . Then
From comparison principle of ODE, we get
This yields
where \lambda_{2} = \min\{1-\frac{\chi^{2}\gamma^{2}}{32\alpha}, \frac{\delta}{3}\} and C_{9} = \max\left\{|\Omega|-\frac{3\chi^{2}\beta^{2}}{32\alpha(3-\delta)-\chi^{2}\gamma^{2}}, \frac{3\chi^{2}\beta^{2}}{32\alpha(3-\delta)-\chi^{2}\gamma^{2}}\right\} .
From the inequalities (3.11), (3.13) and (3.14), we derive
where C_{10} = \max\left\{\frac{2\gamma^{2}}{\alpha^{2}}C_{9}, \frac{2\beta^{2}}{\alpha^{2}}\right\} . By employing the uniform estimation (1.7), the inequalities (3.9), (3.14) and (3.15), the exponential decay estimation (1.10) can be obtained.
The proof is complete.
4.
Concluding remarks
In this paper, we mainly study the uniform boundedness of classical solutions and exponential asymptotic stability of the unique positive equilibrium point to the chemotactic cellular model (1.3) for Baló's concentric sclerosis (BCS). For model (1.1), by numerical simulation, Calveza and Khonsarib in [7] shown that demyelination patterns of concentric rings will occur with increasing of chemotactic sensitivity. By the Theorem 1.1 we know that systems (1.1) and (1.2) are {uniformly} bounded and dissipative. By the Theorem 1.2 we also find that the constant equilibrium point of model (1.1) is exponentially asymptotically stable if
and the constant equilibrium point of the model (1.2) is exponentially asymptotically stable if
According to a pathological viewpoint of BCS, the above stability results mean that if chemoattractive effect is weak, then the destroyed oligodendrocytes form a homogeneous plaque.
Acknowledgments
The authors would like to thank the editors and the anonymous referees for their constructive comments. This research was supported by the National Natural Science Foundation of China (Nos. 11761063, 11661051).
Conflict of interest
We have no conflict of interest in this paper.













 DownLoad:
DownLoad: