1. Introduction
One of the core tasks in biodiversity conservation is to protect and restore populations of endangered species and their habitats. Successful conservation includes the identification of the causes of population decline, and the design and application of actions for restoring more suitable conditions for the populations [1,2]. Causes of decline, or threats, can be identified by analysing the declining populations based on biological and environmental information, such as demography, genetics, habitat conditions, and landscape fragmentation. Such data can usually be sampled in the current populations and their environments. In contrast, there is often less empirical data to support the design of a suitable target habitat, i.e., a more favorable habitat than the current. Frequently, the desirable environmental conditions and demographic properties are no longer present in sub-optimal habitats and declining populations and thus cannot easily be discovered by field studies. Hopefully, viable populations are found elsewhere to serve as blueprints for restoration, but for rare species this may not be the case. Instead, other approaches are needed to identify target conditions for restoration, for example conditions that prevailed at a time before populations began to decline and the species became endangered.
One approach is to use historical sources to track previous environmental conditions [3,4]. For human-made habitats in the agricultural landscape, there may be historical information from different time periods that directly or indirectly describe land-use, for example, cadastral maps, taxation records, and regional sheriffs' reports [5]. Some of these sources have been used in ecology and conservation to relate an ecological variable, usually species richness, to historical land use [6,7,8,9,10,11,12], habitat type [13], or historical habitat connectivity [14,15]. There are considerably fewer studies addressing the species and population levels [10,16,17,18] or habitat processes [19,20]. Several such historical-ecological studies have demonstrated the importance of historical conditions for the conservation of current biodiversity. For example, Gustavsson et al. [7] showed that grassland plant species richness was better correlated with 18th century land-use than with later periods, indicating that conservation management should consider reintroducing elements of that period's grassland management.
The combined use of historical and ecological data requires interdisciplinary collaboration in order to correctly interpret historical sources and to link their information to the ecology of the target species, for example, by translating the historical information into habitat conditions or disturbance regimes [21]. An important aim of such interdisciplinary analyses is to identify those elements of the past that are indispensable for conservation status of species and habitats and to integrate these key elements in conservation measures and land-use [22,23]. The task is challenging because historical sources rarely provide explicit information about such environmental variables that are of key importance for the population viability of species.
The aim of this study is to test a new method for connecting historical information about land-use with the biology of species. We combine biological and environmental information about the life-cycle and habitat of the endangered butterfly, Clouded Apollo (Parnassius mnemosyne L), with the land-use history of its habitats in Sweden, in order to design and experimentally test a management regime aimed at optimizing habitat quality for the species. P. mnemosyne is declining in most of its European range and continues to decline in Sweden despite considerable conservation measures based on the Swedish Action Plan for the species [24,25,26]. Specifically, we investigate:
● If a combination of historical land-use information and species biology data can provide new insights in the causes of decline of the species, compared to the ecological information alone.
● How species-relevant key elements of the historical land-use can be identified and used for designing a target habitat for the species.
● How the species responds to conservation measures that are based on the historical-ecological analysis.
2. Study system and methods
2.1. Study species
Parnassius mnemosyne is a Palaearctic species occurring in scattered local populations, mainly in Europe [24]. It is red-listed by IUCN because of rapid decline in several European countries where it is threatened both by intensified land-use and abandonment [24]. For example, a decline in distribution or population size of more than 30% have been reported from Bosnia and Herzegovina, Germany, Latvia and Ukraine and of 6–30% from Albania, Austria, Belarus, Czech Republic, France, Hungary, Romania and Switzerland [24]. In Sweden P. mnemosyne is classified as endangered [27] and occurs in the agricultural landscape, mainly in remnants of grassland with weak or no management and with a mosaic of trees and shrubs. The larva is monophagous on Corydalis species, in Sweden mainly C. solida and C. intermedia. P. mnemosyne is known from ten Swedish provinces, but became locally extinct in seven of these during the first half of the 20th century [25]. In 2008, when the Swedish Action Plan was set up for saving P. mnemosyne [25,28], it still occurred in three provinces, Blekinge (8 populations), Uppland (8 populations), and Medelpad (25 populations).
2.2. Study area and sites
This study focuses on populations of P. mnemosyne in Uppland in the County of Uppsala in east-central Sweden, where the species occurs in the innermost parts of the Roslagen archipelago in the Baltic sea. The region is 0–30 meters of height above sea level and characterized by an undulating agrarian landscape, mixed with forest and shores. The landscape is a mosaic of arable land on clay-rich soils and pastures and forest patches on low hills with poorer soils built up mainly by till interspersed with bedrock. The soils are calcareous. Agriculture is dominated by small-scale farms and the region has a long history of mixed agriculture combining cropping and livestock production based on grazed and mown grasslands.
Eighteen populations of P. mnemosyne are known from the county, all discovered after 1970 [25]. Four of the populations went extinct around 1980 and surveys made in 1985 and 1994 showed a continued decline [25] Table 1. In 1994, 12 of the 14 remaining sites were unmanaged and all sites were characterised as under succession towards taller vegetation and increasingly dense cover of trees and shrubs. A renewed inventory of all 18 sites performed in 2004, as a part of thus current project, found only five remaining populations [29] (Table 1). In the County of Uppsala, C. solida is the only occurring Corydalis-species.
Table 1. Status 2004 of all 18 known populations of Parnassius mnemosyne in the County of Uppsala, Sweden, and the land-use and openness at the sites.
| Site |
Population status |
2004 |
|
|
Land-use1 |
Openness2 |
| Boda |
< 10 indiv. in 2004 |
Ab |
SO & C |
| Brudskäret |
C. 40 indiv. in 2004 |
P & Ab |
SO & Ar |
| Brunnsvik |
Extinct 2002 |
Mow |
SO |
| Fäholmen |
Extinct 2000 |
Ab |
C |
| Gruvskäret |
Extinct 2005 |
Ab |
C & Ar |
| Gräsö gård |
Extinct 1975-80 |
P |
O |
| Hästhagen |
Extinct 1975-80 |
Ab |
C |
| Kavarö |
Extinct 1980-85 |
Ab |
SO & Ar |
| Klyxen |
Extinct 1985-943 |
Ab |
SO |
| Laduskär |
Extinct 1985-94 |
Ab |
SO & C |
| Nyhem |
Extinct 1975-80 |
P |
SO & C |
| Olasskär |
< 10 indiv. in 2004 |
Ab |
O & C |
| Sandika |
Extinct 1985-94 |
P |
S |
| Sandika Sjögärd/ Sandikaön |
7 indiv. in 2004 |
Ab |
SO |
| Strand |
Extinct 1975-80 |
Ab |
C & Ar |
| Taskan |
Extinct 2002 |
Ab |
C |
| Tuskö |
Extinct 2005 |
Ab |
C & Ar |
| Älgsholmen |
C. 40 indiv. in 2004 |
Ab |
CG |
Notes: 1. Ab = Abandoned former pasture, meadow, or arable field; P = Pasture; Mow = Conservation mowing
2. O = Open (<5% canopy cover); S = Scattered trees (6–20%); SO = Semi-open (21–50%); C = Closed, (>50%);
CG = Closed with gaps; Ar = Open arable land
3. Re-colonised from Brudskäret in 2010, see Figure 2 |
2.3. Methods for combining ecological and historical information
2.3.1. Literature review of the ecology of the species
European scientific papers and conservation reports on P. mnemosyne and its host plant written in English, Swedish, or Norwegian, were reviewed in order to identify:
A. Environmental conditions influencing the different stages in the life-cycle.
B. Present European habitat types, including estimates of their suitability for the species.
C. Causes of threat.
D. Suggested measures for restoration and management.
This information was used to summarize the critical ecological components for the species, i.e., the environmental conditions considered most important for the different life stages and for the species in general. The review includes publications from 1993 to 2017.
2.3.2. Historical and current land-use
For each of the 18 known sites for P. mnemosyne (current and former), historical cadastral maps representing five time periods were interpreted. For the first three time periods we used detailed village maps (of 1:4000 or 1:8000 scale) from 1640–1709 (available for 8 of the sites), 1738–1805 (all 18 sites) and 1846–1892 (11 sites). For the 20th century two economic maps were used, one from 1901-05 (scale 1:20,000) and one from 1953 (1:10,000), of which the latter is based on a detailed aerial photograph. The maps were interpreted in order to identify land use components potentially important for the critical ecological components that were identified through the literature review: type, timing, and intensity of land use, and openness/tree cover.
Type of land use, i.e., arable land, hay meadow and pasture etc., is explicitly shown in the four oldest maps. The 1953 economic map shows arable land, open permanent grassland on former fields, open or semi-open pasture, and forest. Here, we interpreted permanent grassland as pasture, not hay-meadow, since all hay in the study region was at this time produced on arable fields in a rotational mixed cultivation system (see below). After 1953 no maps or other official records of land-use are available. Field inventories from the 1970's and 1980's, as well as the successional stage at the localities in 1994, however, indicate that grazing stopped at the sites during the 1970's and early 1980's [25].
Timing in pastures was determined by the fencing system, as shown in the historical maps [17]. During the studied historical period, livestock grazed most of the landscape while arable land and hay meadows were protected from grazing by fences. In the mosaic landscape in this region it was not possible to fence every field or meadow separately, but larger enclosures were constructed that contained areas of pasture together with the arable fields and meadows. Grazing of pasture fenced with hay meadow began after finishing the hay harvest, around late July. Pasture fenced together with arable fields was grazed after harvest of the fields, i.e., from late August. Pastures not being fenced together with other land-use types were accessible and most likely used for grazing from May. Timing of management of hay-meadows was considered to be early July, according to local traditional date for hay-cutting [30].
By the end of the 19th century a new agrarian system was gradually introduced, in which semi-natural pastures and hay meadows were replaced by fodder cultivated on arable fields in a rotational system. During a 3–5 years period with hay-production on the fields, the pasture within the field enclosure was available for grazing together with the field aftermath from mid-July at the earliest. During the following 3–5 year period of cereal cropping, the pastures were usually not grazed at all.
Management intensity here refers to how much of the biomass had been removed by mowing or grazing by the end of the vegetation period [31]. In hay-meadows, the intensity is high, as all grass is cut at the mowing. For pastures, the grazing intensity is the combined result of land productivity, length of the grazing season, and stocking density [32]. The available information from maps and field surveys does not allow quantitative analysis of these factors, but we combined two indicators to obtain a brief estimate of the P. mnemosyne sites in relation to a grazing intensity gradient:
● Notations in some maps of scarce pasture supply indicate more intense grazing while notations of abundant pasture indicate weaker grazing intensity.
● More intense grazing can be assumed in enclosures with small areas of grassland (i.e., pasture, hay meadow, and cultivated grassland), since most of the biomass could be consumed even by a smaller number of grazers.
Tree cover (openness) was quantified using the aerial photograph in the economic map from 1953. We classified the sites as open (<5% canopy cover), scattered trees (6–20%), semi-open (21–50%), closed (>50%), and closed with gaps. None of the older maps give sufficient information about openness.
The land-use components type, timing, and intensity of current land-use was determined by a field survey of all 18 sites in 2004, together with openness according to the categories listed above.
2.3.3. Combining information about the species' ecology with historical/current land-use
The ecological components identified through the literature review were compared with the land-use components in order to identify positive and negative links between the species' habitat requirements and the different historical and current land-use practices that influence the habitat. Each of the identified land-use components was evaluated against all identified ecological components in terms of likely positive, negative, or neutral effects. The size of the effect was not evaluated.
2.4. Design of restoration and management
Through collaboration with local farmers and the conservation organisation Upplandsstiftelsen, plans for restoration and management were designed and implemented at two of the sites with P. mnemosyne, Boda and Brudskäret. The plans were based on the results of the comparison between species ecology and land-use information, and we aimed at combining as many positive, and omitting as many negative relationships as possible. Restoration measures were performed in the autumn–winter 2004, and the continuous management applied from after the flight period in 2005 and onwards. The responses of the populations of P. mnemosyne and its host-plant were monitored annually in the spring from 2004 to 2014.
The general habitat structure was restored by logging and clearing of certain proportions of the trees and shrubs. Timing of grazing was regulated by fencing of the sites and regulating the opening of fencing. Intensity of grazing was regulated by stocking density and length of the grazing period.
2.5. Response of Parnassius mnemosyne and its host plant, Corydalis solida, to restoration
2.5.1. Parnassius mnemosyne
Relative population sizes were estimated at the peak of the flight period at the two restored localities Boda and Brudskäret and at two non-restored sites, Älgsholmen and Sandika Sjögärd/Sandikaön. Similar to the experimental sites, these control sites were abandoned semi-open pastures in 2004. Their population sizes at the beginning of the study period were similar to the experimental populations (Table 1).
The numbers of flying butterflies were counted during criss-crossing the area in which the species occurred, at a pace of c. 2 km/h, corresponding to 15 min/hectare. All observed butterflies were recorded, thus not only those within a prescribed transect width [33]. In order to minimise double-counting, Boda was divided into two sub-sites of 1.5 hectares each and walked simultaneously by two recorders during 22 min for a total monitoring time of 44 min. Brudskäret was divided into four sub-sites of 1.5 hectares each, which were walked by four persons during 22 min. At Älgsholmen, two sub-sites of 2 hectares were monitored for 30 min, and at Sandika Sjögärd/Sandikaön, two sub-sites of 1.5 hectares were monitored for 22 min. Recording was done only in sunlight, since this was found to be a prerequisite for flight. The sites were visited at several occasions during the flight period and the highest observed number was used, which can be assumed to best reflect the number when all butterflies were hatched. Using the same method, butterflies were also looked for and counted in the terrain around the main sites in order to detect dispersal and establishment of new subpopulations.
The estimate of relative population size, obtained by the monitoring method, indicates the variation between years and the response to management. Since the relative population size is based on the number of flying adults observed at one occasion, it can be assumed to underestimate the actual population size. Warren et al. [33] transformed the number of observed adults to density per unit of monitoring time, i.e., observations/h, and also adjusted for site area by multiplying observations/h with area in hectares. We present both actual observed number, observations/h, and observations/h*ha.
2.5.2. Corydalis solida
The response of the host plant, Corydalis solida, to the restoration and management at Boda and Brudskäret was estimated by counting the number of plants in six 15 × 4 m fixed transects per site. The transects were placed in open grassland. The effects of restoration were compared with three types of control treatments, (a) unmanaged former pasture (3 transects per site), (b) continuous grazing May–October (3 transects), and (c) mid-July mowing followed by grazing from mid-August (4 transects). Unmanaged and continuous grazing were obtained by fencing at each site. The mowing experiment was performed in two fenced 10 × 20 m experimental plots per site.
Because of small sample sizes and unequal variances, non-parametric tests were used to analyse the data. First, a Kruskal-Wallis test was used to test for differences in mean density between treatments. Each site and year was tested separately, based on means of the transects in each treatment. For years with significant difference between treatments, Dunn's test was used for post-hoc testing of pairwise differences, with Bonferroni correction for multiple comparisons. The tests were performed using NPTESTS procedure in SPSS v. 19.
3. Results
3.1. Critical ecological components for Parnassius mnemosyne
We used 20 reviewed scientific papers and conservation reports from seven European countries: The Czech republic, Denmark, Estonia, Finland, Hungary, Norway, Russia, and Sweden (Table 2). We focused the review on studies related to the habitat. Papers dealing with other topics, such as genetics or metapopulation dynamics were reviewed, but only concerning information about habitat and life cycle parameters. Some publications mention, study, or identify critical ecological factors for specific stages of the life cycle, although most studies address the ecology of the adult stage only. Some publications describe threats and conservation measures for the species more in general. Most studies describe in general terms the habitats in which P. mnemosyne has been observed and studied, but only a few studies analyse the importance of specific habitat structures and management components. Recommendations for management measures can be found in many of the publications.
Table 2. Identified critical biological and environmental factors and their effects (A), important habitats (B), major threats (C), and suggested management measures (D), all related to specific stages in the life cycle of Parnassius mnemosyne or the species in general. Phenology refers to Swedish conditions.
| A. Critical factors and direction of effect (+/−) |
B. Habitats |
C. Threats |
D. Suggested management |
| P. mnemosyne in general |
● Managed grasslands and previously managed grasslands mixed with trees and bushes [25,34,35,37,38,41,46,47,48,50,51,52]
● Forest with man-made gaps in succession [36,43,44,45,49]
● Forest with naturally made gaps [34,35] |
● Grazing, especially grazing before 31 July and sheep grazing [25,35,39]
● Succession of grasslands due to ceased traditional management practices (mowing and grazing) [35,46,47,48,50,51,52]
● Succession of gap-forest into even-aged forest, due to ceased traditional forest practices and coppicing [42,49]
● Changed forest composition, from deciduous to coniferous, due to changed forest management [45,49] |
● Manual clearing of bushes and trees in grasslands, no grazing [39]
● Grazing, but regulated regarding timing, intensity (extensive) and livestock, in order to keep the grasslands open and prevent succession [25,35,39]
● Regular small-scale forest clearing (coppicing) in order to always have habitat in the right successional stage [36,42] |
| Larva (April) |
|
|
|
| + Sufficient Corydalis sp. for feeding [25,39,51] 50 shoots per larva or 3 m2 per larva [51]
+ Warm microsites (c. 26 ℃) for digestion and development [52] |
●
C. intermedia: Light gaps in forest gaps, sunny margins of (deciduous) forest and in open grassland [35,40]
● C. solida: deciduous forests and open or semi-open grasslands. Sunny margins of forest and trees. Forest clearings and closed canopy [42,44,46,52]
● Larva: Open meadows with both sunny spots and some litter [52] |
● C. intermedia: Succession to forest [40]
● Larva: Increased canopy cover, causing slower larval development [52]
● Larva: Intense grazing, can remove larva sitting on vegetation [39] |
● Preserve open meadows with Corydalis and management to prevent natural succession [51] |
| Pupa (May) |
|
|
|
| + Dry leaves in dry place for attaching pupa [25,52]
+ warm microsites for development [52] |
|
● Intense grazing: pupa can be crushed by trampling of grazing animals [39]
● Increased canopy cover, causing slower pupal development and increased mortality [52] |
|
| Imago (June–July) |
|
|
|
| + Nectar flowers for feeding [25,42,48]
+ sunny and wind protected place for mating and feeding [43,50,51]
+ sites with Corydalis bulbs close to bushes, for oviposition [37,42] |
● Flower rich habitat, providing pollen for the imago [34,46,50] |
● Grazing, removes nectar providing flowers [35] |
|
| Egg (July–April) |
|
|
|
|
|
● Risk with intense grazing: removal of eggs attached to vegetation and trampling can crush the eggs [25,39] |
● Avoid grazing or very careful grazing in order not to lose eggs [25,39] |
We were able to identify critical ecological components for all life stages of P. mnemosyne and found no conflicting conclusions among the reviewed literature regarding these components (Table 2, Column A). There was a greater discrepancy between studies regarding which types of habitat management that promotes these ecological components (Table 2, Column D), and also regarding causes of threat (Table 2, Column C).
In summary, the general habitat structure for P. mnemosyne can be described as a mosaic of open grassland and trees/shrubs/forest, providing Corydalis spp. in spring and nectar plants in early summer (Table 2, Column B). This habitat is shaped by some kind of disturbance, usually anthropogenic, which either counteracts overgrowth and maintains grassland with trees and shrubs, or generates a continuous flow of suitable successional gaps in forest. The disturbance to the grassland vegetation needs to be of a type that does not remove or damage too much of the larval host plants, nectar flowers, eggs, larvae, or pupae. Specifically, the habitat should contain the following ecological components, of critical importance for P. mnemosyne:
1. Open grassland that provides:
a. Flower-rich grassland vegetation for the nectar-feeding imago.
b. Sun exposed habitat for sufficient temperature in spring and early summer for larva, pupa, and imago.
2. Presence of deciduous trees and shrubs that provides:
a. Suitable growth conditions for Corydalis plants for the larva.
b. Wind shelter for the feeding and mating imagoes.
c. Dry leaves to which the pupa is attached.
3. Habitat management that provides:
a. Low enough vegetation and litter to allow sufficient exposure and day temperatures close to the ground for the larva.
b. Undisturbed conditions in April–May for host plant, larva, and pupa.
c. Undisturbed conditions in June-early July for the imago.
d. Very restricted biomass removal July–April in order to not remove eggs attached to the vegetation.
3.2. Historical an current land-use components at the sites
Type of land-use. The historical land-use type was pasture at all 18 sites for P. mnemosyne in the County of Uppsala, through all time periods possible to analyse, i.e., from the mid 17th century until the management ceased in the 1970's or 1980's (Table 3).
Table 3. Type and timing of land-use during five historical time-periods at 18 sites for the Clouded Apollo, Parnassius mnemosyne, in the County of Uppland, Sweden, based on historical land-use maps (see methods description). Land-use type and fencing arrangement are explicitly noted in the maps, and used for interpretation of time for earliest land management in the summer, and of the intensity of management.
| Site |
1640–1709 |
1738–1805 |
1846–1892 |
1901–1905 |
1953 |
|
Land-use (ha)1 |
Timing2 |
Open-ness3 |
Land-use (ha)1 |
Timing2 |
Open-ness3 |
Land-use (ha)1 |
Timing2 |
Land-use1 |
Timing2 |
Land-use1 |
Timing2 |
Open-ness4 |
|
Type |
Fenced with |
|
|
Type |
Fenced with |
|
|
Type |
Fenced with |
|
Type |
Fenced with |
|
Type |
Fenced with |
|
|
| Boda |
|
|
|
|
P(2) |
M(3) Ar(0.5) |
July/Aug |
|
P(2) |
M(4) Ar(0.5) |
July/Aug |
P |
M & Ar |
July/Aug |
P |
|
May |
SO |
| Brudskäret |
P |
|
May |
|
P(21) |
M(9) |
July |
Sp |
|
|
|
P |
M & Ar |
July/Aug |
P |
Ar |
July |
S & Ar |
| Brunnsvik |
P(45) |
|
May |
Sp |
P(25) |
|
May |
Sp |
P(25) |
|
May |
P |
|
May |
P |
|
May |
SO |
| Fäholmen |
|
|
|
|
P(19) |
M(18) |
July |
Sp |
P(?) |
M(?) Ar(?) |
July/Aug |
P |
M & Ar |
July/Aug |
P |
Ar |
July |
O & SO |
| Gruvskäret |
P(115) |
M(10) |
July |
Sp |
P(110) |
M(20) |
July |
Sp |
P(105) |
M(25) |
July |
P |
M & Ar |
July/Aug |
P |
Ar |
July |
SO & Ar |
| Gräsö gård |
|
|
|
|
P(12) |
|
May |
|
|
|
|
P |
|
May |
P |
|
May |
O |
| Hästhagen |
|
|
|
|
P(9) |
M(7) |
July |
|
P(9) |
M(7) |
July |
P |
M & Ar |
July/Aug |
P |
Ar |
July |
SO & C |
| Kavarö |
P(11) |
Ar(7) |
Aug |
|
P(10) |
M(1) Ar(7) |
July/Aug |
|
P(?) |
Ar(?) |
Aug |
P |
Ar |
Aug |
P |
Ar |
July |
SO |
| Klyxen |
P(200) |
M(15) |
July |
Sp |
P(200) |
M(15) |
July |
Sp |
|
|
|
P |
M & Ar |
July/Aug |
P |
Ar |
July |
O & SO |
| Laduskär |
|
|
|
|
P?(6) |
|
May |
|
P(3) |
M(3.5) |
July |
P |
M |
July |
P |
|
May |
SO & C |
| Nyhem |
|
|
|
|
P(2) |
M(2) |
July |
Sp |
|
|
|
P |
Ar |
Aug |
P |
Ar |
July |
S & SO |
| Olasskär |
P(5) |
M(5) |
July |
|
P(7) |
M(4.5) |
July |
Sp |
|
|
|
P |
M & Ar |
July/Aug |
P |
|
May |
O & SO |
| Sandika |
|
|
|
|
P(4) |
M(2.5) Ar(3) |
July/Aug |
|
P(5) |
Ar(6) |
Aug |
P |
M & Ar |
July/Aug |
P |
Ar |
Aug |
S & SO |
| Sandika Sjögärde |
|
|
|
|
P(33) |
M(2) Ar(11) |
July/Aug |
|
P(33) |
M(02) Ar(20) |
July/Aug |
P |
Ar |
Aug |
P |
Ar |
Aug |
SO |
|
Land-use (ha)1 |
Timing2 |
Open-ness3 |
Land-use (ha)1 |
Timing2 |
Open-ness3 |
Land-use (ha)1 |
Timing2 |
Land-use1 |
Timing2 |
Land-use1 |
Timing2 |
Open-ness4 |
|
Type |
Fenced with |
|
|
Type |
Fenced with |
|
|
Type |
Fenced with |
|
Type |
Fenced with |
|
Type |
Fenced with |
|
|
| Strand |
|
|
|
|
P(4) |
M(6) |
July |
Sp |
|
|
|
P |
M & Ar |
July/Aug |
P |
Ar |
July |
SO & Ar |
| Taskan |
|
|
|
|
P(5) |
|
May |
|
P (8) |
|
May |
P |
|
May |
P |
|
May |
O & SO |
| Tuskö |
P(120) |
Ar(20) |
Aug |
Sp |
P(115) |
Ar(25) |
Aug |
O or Sp |
P(115) |
Ar(25) |
Aug |
P |
Ar |
Aug |
P |
Ar |
July |
SO & Ar |
| Älgsholmen |
P |
M |
July |
|
P(8) |
M(12) |
July |
Sp |
|
|
|
P |
M |
July |
P |
|
May |
O & SO |
Notes: 1. P = Pasture; M = Meadow; Ar = Arable field
2. Timing refers to earliest possible management based on the fencing arrangement, see text for explanation. July/Aug denotes grazing from August except for years of fallowing when the pastures were available from July.
3. Based on surveyor information: O = open (no trees according to the surveyor); Sp = Sparse tree cover; C = Closed (dense forest)
4. Based on aerial photographs: O = Open (<5% canopy cover); S = Scattered trees (6–20%); SO = Semi-open (21–50%); C = Closed, (>50%); Ar = Open arable land |
Timing of land-use. 15 of the 18 pastures had a history of being grazed late, either starting from late July or late August (Table 3, periods 1738–1805 and 1846–1892). All these 15 pastures remained grazed from either late July or late August until the 1901-05 time-period. At sites for which older data were available, this fencing arrangement existed also during the first time-period, 1640–1709, except for one site, Brudskäret, which changed from May– to July– grazing between the first and second time period (Table 3).
During the 20th century, four of the 15 previously late-grazed sites became early-grazed pasture, while 11 of the sites continued to be fenced with arable fields and thus obtained a shifting grazing rhythm with late grazing alternating with no grazing, as explained in the methods (Table 3). Three of the 18 pastures were grazed early (from May) throughout all historical time periods (Table 3).
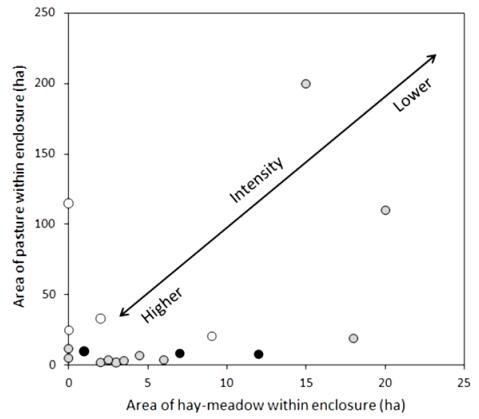
Management intensity. The grazing at the P. mnemosyne sites was most likely relatively intense. The area of pasture and meadow at the 18 sites in the 1738–1804 period (Table 3) are summarised in Figure 1. 1738–1804 is the only time period with maps with notations on pasture availability from all studied villages. With few exceptions, P. mnemosyne occurred in fenced pastures which were small in comparison to the up to 200 ha outland pastures used by the villagers. A majority of the sites had a relatively small area of both pasture and hay meadow (Figure 1). Most of the sites are thus positioned towards the small area/intense grazing- end of the gradient in Figure 1. Historical qualitative information about the pasture abundance was available for seven of the sites only. For four sites the surveyor stated pasture deficit in the village and for three sites abundant pasture.
Trees and shrubs. The 1953 economic map, with an aerial photograph from 1941, showed that 16 of the 18 sites were semi-open (Table 3). Two sites were entirely open or with scattered trees. In earlier maps with surveyors' entries on tree cover, a sparse cover is by far the most common description.
Land-use 2004. Of the 18 sites, 13 lacked management, four were grazed from May to October, and one site was mown in late July for conservation purposes (Table 1).
The cover of trees and shrubs had increased since the 1941 aerial photograph. On six of the sites the entire pasture had a closed cover, and on another three sites parts of the pasture were overgrown (Table 1). Six sites had a semi-open cover or scattered occurrence of trees.
The above interpretation of historical and current land-use identifies number of land-use components, is summarised in Table 4. The directions of the relationships were usually obvious.
Table 4. Analysis of how different components of land-use, in 2004 and historically, can be expected to influence a number of ecological components, of critical importance for Parnassius mnemosyne, see text for explanation.
| Land-use components 2004 |
Ecological components of critical importance for Parnassius mnemosyne imago (I), egg (E), larva (L), or pupa (P) |
Historical land-use components (1640–1905) |
| Abandoned pasture |
Semi-natural pasture |
Semi-natural pasture |
| Tall, undisturbed vegetation |
Increasing and often closed cover of trees and shrubs |
Rather intense grazing from May |
Grazing from May |
Grazing from late July or mid-August |
High to moderate grazing intensity from July–August |
Mosaic of grassland, trees, and shrubs |
3–5 yr. periods without grazing (20th century) |
| - |
- |
+ |
(1a) Flower-rich grassland vegetation (I) |
+ |
+ |
+ |
+ |
? |
| 0 |
- |
0 |
1b) Sun exposed habitat for sufficient temp. in spring-early summer (I, L, P) |
0 |
0 |
0 |
+ |
0 |
| +
/-1 |
+ |
- |
(2a) Suitable growth conditions for Corydalis (L) |
- |
+ |
0 |
+ |
? |
| 0 |
+ |
0 |
(2b) Wind shelter for feeding and mating (I) |
0 |
0 |
0 |
+ |
0 |
| 0 |
+ |
0 |
(2c) Dry leaves (P) |
0 |
0 |
0 |
+ |
0 |
| - |
- |
+ |
(3a) Low enough vegetation and litter to allow sufficient exposure and ground temperature (L) |
+ |
+ |
+ |
0 |
? |
| + |
0 |
- |
(3b) Undisturbed conditions in April–May (L, P) |
- |
+ |
0 |
0 |
+ |
| + |
0 |
- |
(3c) Undisturbed conditions in June (I) |
- |
+ |
0 |
0 |
+ |
| + |
0 |
- |
(3d) Restricted biomass removal July–April (E) |
- |
- |
- |
+ |
+ |
| Notes: 1. Initially Corydalis may be favoured, but eventually the grass litter layer will be too thick and the forest too dense. + = positive effect, − = negative, 0 = neutral effect, and? = unclear effect. |
3.3. Comparison between ecological components and land-use components, design of restoration and management
3.3.1. Current land use
In 2004, the sites constituted either abandoned former pasture or pasture grazed from the early summer. Early grazing can be assumed to have negative effects on several of the necessary ecological components, since both P. mnemosyne and its host-plant require undisturbed conditions early in the season (Table 4). In abandoned pastures this requirement is fulfilled, but instead other critical ecological components are disfavoured by tall vegetation and closed canopy, such as the needs for flower-rich vegetation and sun-exposed, warm microhabitats (Table 4).
3.3.2. Historical land use
The historical land-use, in contrast, showed more positive relationships between land-use components and the critical ecological components. Late-season grazing in mosaic grassland likely provided the necessary warm microclimate, a flower-rich vegetation and undisturbed conditions in April–June (Table 4). A rather intense grazing favours species-rich grassland vegetation by reducing the dominance of tall competitive plants and keeping the litter layer thin. Only one of the identified critical ecological components, the need for minimising removal of eggs attached to the vegetation, was obviously disfavoured by the historical grazing regime. The presence of shrubs, however, would have reduced this risk, as well as the risk of damage to larvae and pupae, because shrubs can constitute grazing refugia. Three of the 18 sites were historically grazed from May, and can thus be assumed to have been less suitable for P. mnemosyne (Table 4), especially in the two small pastures, Taskan and Laduskär (Table 3).
The rotational land-use during the 20th century added one land-use component, namely 3–5 year periods without management (alternating with grazing from July). Such periods would have reduced the average biomass removal and thus the risk of removing eggs, but unmanaged periods would have, on the other hand, accumulate litter and increase the average vegetation height, thereby affecting the microclimate for larvae and pupae negatively. It is unclear if 3–5 years without management is enough to significantly influence the larvae, pupae, Corydalis, or the grassland species richness and abundance of nectar plants (Table 4).
3.3.3. Design of restoration and management
Based on the comparison between critical ecological components for the species and land-use components the suitable habitat for P. mnemosyne in the County of Uppsala can be described as: A semi-open semi-natural pasture grazed fairly intensely from late July or late August, sun-exposed but with sheltering deciduous trees and shrubs. The grazing may be replaced by July-mowing, provided that Corydalis is favoured by mowing.
The structure of this target habitat was restored at the two sites, Boda and Brudskäret, by logging a proportion of the tall shading trees but keeping a mosaic of sheltering shrubs in the grassland, and by favouring deciduous trees and shrubs at the cost of coniferous trees. The historical grazing from July–August was re-introduced by fencing of grazing areas that contained both pasture hills (being the sites for P. mnemosyne) and surrounding arable fields used for hay production. At onset of grazing in late July, the livestock (cattle) were allowed to choose freely between the pasture hills with P. mnemosyne and the aftermath on the arable fields until the end of the grazing season in late October.
3.4. Response of Parnassius mnemosyne and its host plant
3.4.1. Parnassius mnemosyne
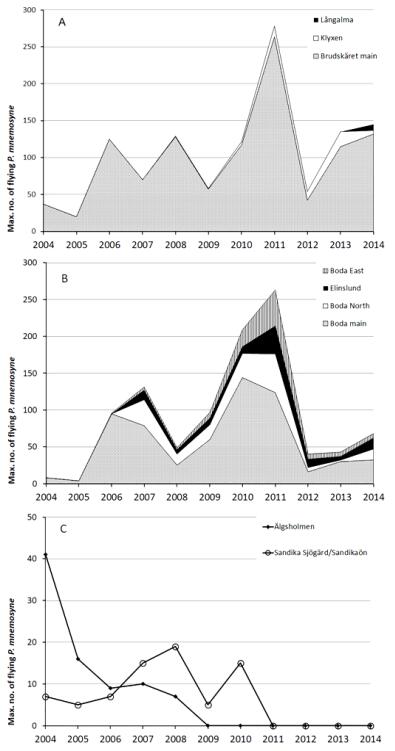
At both sites, the new management regime, i.e., late grazing, proved to be successful for the P. mnemosyne populations. Population numbers increased after only one summer of late grazing, compared to numbers recorded the two years before restoration (Figure 2). At Brudskäret the increase was c. four times (from 24.7 and 13.3 to 83.3 observations per hour, Table 5), at Boda c. 15 times (from 10.7 and 5.3 to 126.7 observations per hour, Table 5). During the following years the populations varied considerably, but remained at a considerably higher level than before restoration. The variation was partly synchronous between the two sites due to weather variation, partly asynchronous.
Table 5. Density per unit time (observations/hour) of Parnassius mnemosyne at four sites in the County of Uppsala, Sweden. The sites Boda and Brudskäret are divided into sub-sites.
| Year |
Observations per hour |
|
Boda |
Brudskäret |
Älgsholmen |
Sandika Sjögärd/Sandikaön |
|
Boda main |
Boda North |
Elinslund |
Boda East |
Brudskäret main |
Klyxen |
Långalma |
|
|
| 2004 |
10.7 |
0 |
0 |
0 |
24.7 |
0 |
0 |
41.0 |
9.3 |
| 2005 |
5.3 |
0 |
0 |
0 |
13.3 |
0 |
0 |
16.0 |
6.7 |
| 2006 |
126.7 |
0 |
0 |
2.7 |
83.3 |
0 |
0 |
9.0 |
9.3 |
| 2007 |
105.3 |
46.7 |
34.7 |
10.7 |
46.7 |
0 |
0 |
10.0 |
20.0 |
| 2008 |
33.3 |
20.0 |
16.0 |
8.0 |
85.3 |
1.3 |
0 |
7.0 |
25.3 |
| 2009 |
80.0 |
26.7 |
24.0 |
21.3 |
38.0 |
1.3 |
0 |
0 |
6.7 |
| 2010 |
192.0 |
44.0 |
24.0 |
61.3 |
78.0 |
6.7 |
0 |
0 |
20.0 |
| 2011 |
165.3 |
69.3 |
101.3 |
130.7 |
176.0 |
18.7 |
0 |
0 |
0 |
| 2012 |
21.3 |
8.0 |
29.3 |
18.7 |
28.0 |
16.0 |
0 |
0 |
0 |
| 2013 |
40.0 |
2.7 |
13.3 |
16.0 |
76.7 |
26.7 |
0 |
0 |
0 |
| 2014 |
42.7 |
20.0 |
40.0 |
16.0 |
88.0 |
6.7 |
10.7 |
0 |
0 |
In contrast, the two populations without the new management regime declined during the study period and eventually went extinct, Älgsholmen in 2009 and Sandika Sjögärd/Sandikaön in 2011 (Figure 2). These sites had a slightly denser cover of trees and shrubs than the experimental sites and, since they were grazed with very low intensity or not at all, considerably taller ground vegetation and deeper litter layer.
Estimates based on observed numbers instead of observations/hr change the differences in population size, but not the variation over time within sites.
3.4.2. Corydalis solida
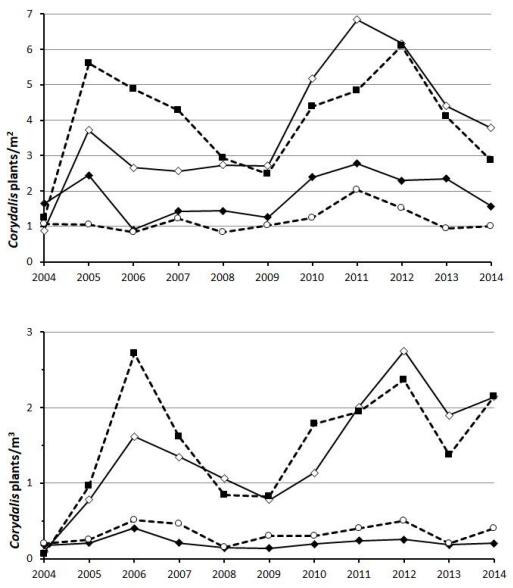
At both experimental sites, Corydalis solida responded positively to late disturbance, both where grazing from late July to October, and where mowing in mid-July followed by aftermath grazing from mid-August was applied (Figure 3). Grazing from May to October and no management at all both supported considerably lower host plant densities. Densities varied between treatments all years (Kruskal-Wallis p < 0.01). In both Brudskäret and Boda, the treatment no management showed significantly lower density than late grazing and mowing all years (Dunn-Bonferroni test, p < 0.05), but did not differ from the treatment grazing May–October in any year (p > 0.15). In Boda, grazing May–October also differed from the treatments late grazing and mowing in all years, and in Brudskäret, all years (p < 0.05) except for 2005 and 2014. The treatment late grazing never differed from mowing at any of the sites (p > 0.1). The density varied between years more or less synchronously between management regimes and to some extent also between sites (Figure 3).
4. Discussion
The Swedish Action Plan for rescuing Parnassius mnemosyne is based on knowledge about its present habitat in Sweden and the biology of the species [25]. This information indicates two contradicting demands: the need for disturbance to maintain sun-exposed grassland patches and the species' sensitivity to grazing. In the Action Plan these management contradictions are handled by recommending regular clearing of shrubs and trees in order to maintain a mosaic tree-grassland habitat, and cautious mowing, preferably without removing the cut grass, which may contain eggs. Grazing should be avoided in most populations until they have recovered [25]. An evaluation of the Action Plan has, however, shown limited success of these measures [26]. In this study, the historical sources confirmed the assumption of a mosaic habitat, but also revealed a historically common grazing regime, rather intense grazing from late July or late August, which is no longer present at any of the sites and which can thus not be discovered by field studies only. An historical-ecological analysis of this grazing regime suggested that it would provide most of the critical ecological components identified for the species. When applied, this management strategy led to increased population sizes of P. mnemosyne and its host plant, Corydalis solida. To our knowledge, this is the first study to perform a detailed analysis of the links between land-use history and a species' ecology, and to specifically study the effects of habitat management on P. mnemosyne.
4.1. Response of Parnassius mnemosyne and its host plant
For economic reasons, it was not possible to perform full-scale management experiments on more than two sites, and to monitor more than two additional control sites. Although acknowledging the limitations of the small sample, we find it likely that the observed positive effects on P. mnemosyne actually show a response to restoration and management. First, the population sizes were considerably larger all years after introducing the grazing regime than the two years prior to restoration. This is unlikely to be a weather effect since the weather in 2004 and 2005 is not considered to be exceptionally problematic for butterflies in Sweden [53,54]. Second, the two populations not subject to the experimental management regime, but to weak grazing and shrub clearing, continued to decline after 2005, and eventually went locally extinct. Third, the increase of host-plant density in response to late grazing offers one causal explanation for the increase of P. mnemosyne.
The identified historical management regime, late, rather intense grazing, was positively related to all critical ecological factors except for the tentative need for protection of eggs attached to the vegetation. The positive response of P. mnemosyne to rather intense biomass removal suggests that the effect of grazing on the eggs may be limited, either because larval survival and growth is a more important bottleneck for population viability than egg survival, or because most eggs escape the grazing, for example close to shrubs, which may serve as grazing refugia [55].
The response of Corydalis solida clearly demonstrates the positive effect of late grazing on the food supply for the larva. Compared to unmanaged conditions, late grazing reduces light competition by reducing the litter depth. Compared to grazing from May, late grazing reduces disturbance by the absence of grazing and trampling during the growth period in the spring. Intense spring grazing may also be directly negative for the butterfly because of increased mortality of larvae and pupae.
Many of the habitat components identified as critical for P. mnemosyne have also been identified as critical for other species of butterflies, such as abundance of larval host plants, availability of nectar for adult butterflies, and vegetation structure [56,57,58,59].
All studied sites historically constituted pastures, but often with adjacent meadows. If populations of P. mnemosyne occurred historically in meadow, they may have escaped into late grazed pastures when the meadows were transformed into arable fields during the 20th century. In order to evaluate whether mown meadows may have been a historical habitat for P. mnemosyne we tested mowing with aftermath grazing with respect to effects on the host plant. Mowing had the same effect on Corydalis solida as late grazing, showing that meadows cannot be excluded as P. mnemosyne habitat, provided that mowing doesn't remove too many of the eggs attached to the vegetation.
The density of Corydalis varied over the study period. The variation was to some extent synchronous in Boda and Brudskäret, which probably reflects weather variation. Lower densities in 2008 and 2009 coincided with a small population size of P. mnemosyne in Boda but not in Brudskäret. This difference between sites may be caused by the fact that the host-plant density at Brudskäret was three times higher than in Boda. It is therefore possible that a threshold in host plant density for the butterfly was reached in Boda, but not in Brudskäret [60]. The year 2012 was an unusually cold and rainy spring and summer and a very poor year for butterflies in most of Sweden [61]. P. mnemosyne recovered faster after 2012 in Brudskäret than Boda which may also be related to the density of food plants.
4.2. The historical-ecological approach in conservation planning
The historical ecology framework stresses that many landscapes and their contents of habitats and elements are characterized by the dynamic interactions between humans and nature, and therefore cannot be understood without knowledge of the history of these interactions, for example the land-use history and its underlying socioeconomic context [4,62,63,64,65]. The framework has been used to address several specific questions in restoration ecology [16,66,67,68,69,70], although rarely at the species level [10].
In the case of P. mnemosyne, it seems likely that the species has expanded its geographic and ecological ranges along with the expansion of pre-industrial agriculture, and that it now declines as the traditional practices become abandoned. Pre-industrial agriculture is characterized by a dependence on semi-natural, unfertilized pastures and hay meadows for feeding the livestock [5], and agriculture has therefore opened the originally forested landscapes, thereby creating new niches for the species [71]. In pre-human landscapes, the species may have been restricted to habitats that naturally maintained a certain degree of openness, such as rocky areas [72], steep slopes with frequent landslides [35], or wind-damaged coastal forests and scrublands [25,73]. Human-made sun exposed and warm habitats may be particularly important at the northern borders of the species' range distribution, such as in Sweden [38]. Suitable habitats for P. mnemosyne were formed through agriculture in regions where certain combinations of ecological, topographic, and socio-economic factors interacted to form fine-scaled mosaics of grassland, fields, and deciduous forest, used for hay making, moderately intense or late grazing, occasional burning and logging, coppicing and other leaf harvest etc. With the introduction of mineral fertilisers during the 20th century, fodder production on arable land have largely replaced semi-natural meadows and pastures, which have reverted into forest or been transformed to arable fields. Interestingly, the last populations of P. mnemosyne in Sweden are found in marginal agricultural regions where this transformation has been slower due to topographic and socio-economic constraints.
4.2.1. The need for historical information in biodiversity conservation
The most important threats to small populations are not necessarily the same threats as those that sent the populations into decline [74]. In small populations, threats related to the smallness per se are common, such as environmental and demographic stochasticity [75,76,77] and reduced genetic diversity [78]. Furthermore, the environmental and demographic factors controlling viability of small and declining populations may be different from those being most significant in growing populations. For example, remnant plant populations in suboptimal habitats often show little or no recruitment, but their viability rely on the survival of established plants. Demographic analyses of such populations may underestimate the importance of recruitment when no recruitment is observed and therefore may fail to identify conservation measures that lead to population growth [79]. This study of P. mnemosyne indicated that cessation of a specific grazing regime, late, rather intense grazing, has been a primary cause of decline long before environmental changes related to overgrowth, shade, and management stochasticity became significant threats.
Parnassius mnemosyne is a representative of a large group of species that occur in man-made habitats and need continued habitat management, but that are disfavoured by most types of modern management, often also including management for conservation. Although ceased traditional management is a major threat to biodiversity in the agricultural landscape, a growing body of literature indicate that many species decline in spite of continued management [80,81]. One reason is probably that the current management differs too much from the historical management that once shaped the habitats. In particular, intense and early grazing, and too ambitious bush clearing in pastures appear to be important threats. Analysing 36 action programs for 63 redlisted species in the agricultural landscape, Lennartsson [82] found that intense and early grazing are as severe threats as abandoment. Unsuitable management may be caused by improper criteria for management in agri-environment schemes [83].
In cases when conservation and policy seem to implement habitat restoration and management that do not give desired positive effects on threatened biodiversity, it is necessary to look for alternative conservation measures. This study, together with many others, suggests that important guidance to conservation can be found in the historical use of the landscape.
4.2.2. Identifying and applying historical land-use
Biodiversity at a certain place and time is usually a legacy of the more or less distant past. This has long been acknowledged for long-lived plants, especially trees, which in the cultural landscape can constitute a biocultural heritage from past land-use and other human activities [84,85]. Recent studies have shown that occurrences of short-lived species, such as butterflies, may also be a legacy of historical landscapes and land-use [86]. A consequence of the influence of history on current populations is that conservation that resembles historical management regimes can be expected to do a better job for species that have so far not responded positively to conservation measures. Past conditions are, however, rarely possible (or desirable) to reconstruct as a whole, but we need to identify those details of historical conditions that prove to be crucial for population viability [22]. Such details, or management components, may be certain land-use activities (such as mowing of hay meadows or grazing of pastures), or even certain components of those activities (such as the intensity or timing of grazing, and the frequency of pollarding of trees). With proper knowledge of the necessary ecological functions of these land-use activities of the past, they can then be reintroduced or imitated by using modern methods, and if necessary modify the individual historical components to fit changed environmental and societal conditions [21].
Which land-use components are critical for a species may differ between populations depending on their history and current environment. In this study, late and intense grazing was a successful management regime for P. mnemosyne in the county of Uppsala and we expect this management regime to also function in other Swedish populations having a similar land-use history. A study in Blekinge, southeast Sweden, found that sites with low intensity grazing after June 15 had higher populations of P. mnemosyne than sites with no grazing, earlier grazing, or higher grazing pressure after June 15 [87]. For other European regions having different land use history, historical-ecological analyses would identify other sets of land-use components that could potentially account for the critical ecological components for the species. For example, Benes et al. [36] suggested that a re-introduction of the historic management regime coppicing would favour P. mnemosyne and other butterfly species in Milkovicky woods, Czech Republic. Several other of the cited studies suggest measures for habitat restoration and management, based on positive relationships between environmental factors and occurrence or population size of the species (Table 2). In particular, logging and clearing of overgrown former grasslands are assumed to favour the species, and some studies also stress that grazing or mowing is needed to ensure long-term effect of the clearings e.g., [35,46,48,50,88]. None of the studies have, however, tested different activities or applied the recommendations in practice. One study has evaluated timing of grazing but not separated effects of timing from effects of grazing intensity [87].
Other field studies of P. mnemosyne have identified intense grazing as an important cause of decline based on the observation that survival was better in non-grazed grasslands [25,35,39]. This has underpinned the general view that the species prefers successional habitats with little disturbance to the vegetation. In contrast, our ecological interpretation of the species' historical landscape suggests that P. mnemosyne in this region is connected to intensely used grasslands, rather than to successional habitats. The differing results of different studies highlight the need for identification of management regimes at a necessary level of detail. Grazing is frequently used as a method for maintaining and restoring semi-natural grassland, but the effects of the measures on butterflies are highly varying from positive to negative [57,87,89,90]. One reason for differing results is probably that the general regime "grazing" consists of a range of land-use components related to timing, intensity, type of livestock etc., which can be applied in various combinations. Our analysis shows that grazing may well be organized in ways that includes land-use components being negatively related to critical ecological components for the species. For example, intense grazing from May risks causing decline of the populations due to negative effects on both the food plants and the larvae and pupae (Table 4). It is thus crucial to identify the specific type of grazing, here late and rather intense, which fits the ecology of the species. Furthermore, the management regime interacts with other habitat conditions, and intense late grazing may not be successful in all P. mnemosyne habitats, for example those without shrubs, which may lack grazing refugia for the eggs.
Historical-ecological analyses can preferably be accompanied by empirical testing, such as in this study. Lennartsson and Oostermeijer [16] tested different types of grassland management on a threatened plant species and found the traditional practice to be by far more beneficial than the prevailing management in conservation and agriculture.
The historical-ecological approach requires interdisciplinary co-operation, since it is necessary to interpret both ecological data and historical sources at a necessary level of detail. For example, historical cadastral maps can reveal considerably more, and more reliable, information than is usually derived in ecological studies, if interpreted using an agrarian history source critical framework.
4.2.3. Concluding recommendations
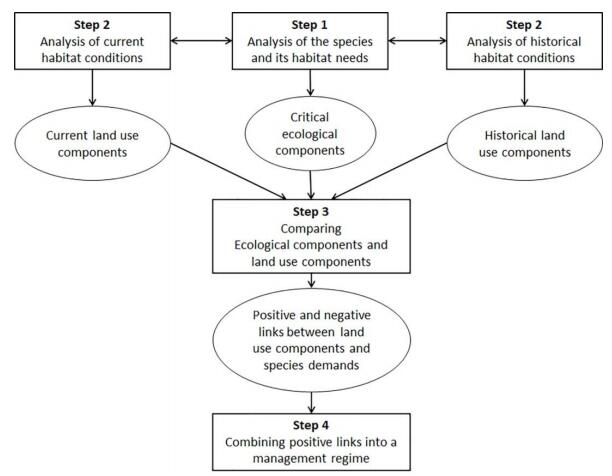
In this study, we applied a systematic method for analysing the causal links between, on the one hand, a species' ecology and habitat needs, and, on the other hand, the current as well as historical land-use. This method can be schematically illustrated as in Figure 4. The analysis aims at understanding how critical ecological components for the target species can be promoted by a set of specific land-use components, and how all these components can be achieved in practice through management regime. A central element of the method is to interpret the species' biology in a historical context and the historical data in relation to the species' biology. In order to identify management components and other environmental variables at a level of detail relevant for the target species, we decomposed the species' biology into its life cycle. This can be recommended as it facilitates the sorting of information, highlights knowledge gaps, and enables a synthesis of habitat requirements for the species as a whole [91].
5. Conclusion
To conclude, this study shows that key components of the species' habitat may be found in historical sources if those are interpreted in an ecological context, and that key measures for successful conservation may be hidden in the historical land-use. We believe that the suggested method would be useful for identifying suitable habitat management in all types of species conservation.
Acknowledgements
We thank Upplandsstiftelsen and the Swedish National Heritage Board for financial support and cooperation, and the land-owners at Boda and Brudskäret for allowing research on their land. We also thank three anonymous reviewers for valuable comments on the manuscript.
Conflict of interest
All authors declare no conflict of interest in this paper.
Sources
Cadastral maps from the villages: Boda, Gräsö, Kavarö, Kulle, Långalma, Norrharg, Sandika, Söderby, Tuskö, Västra Tvärnö, Yttersby and Östra Tvärnö. Situated in Börstil, Gräsö and Harg parishes
Häradskarta, and economic map covering Börstil, Gräsö and Harg parishes









 DownLoad:
DownLoad: