|
[1]
|
Alexander M, Loehr RC (1992) Bioremediation review. Science 258: 874.
|
|
[2]
|
Prasad MN, Prasad R (2012) Nature's cure for cleanup of contaminated environment—a review of bioremediation strategies. Rev Environ Health 27: 181–189.
|
|
[3]
|
Vidali M (2001) Bioremediation. an overview. Pure and Applied Chemistry 73: 1163–1172. doi: 10.1351/pac200173071163

|
|
[4]
|
Gaur N, Flora G, Yadav M, et al. (2014) A review with recent advancements on bioremediation-based abolition of heavy metals. Environ Sci Process Impacts 16: 180–193. doi: 10.1039/C3EM00491K

|
|
[5]
|
Atlas RM, Hazen TC (2011) Oil biodegradation and bioremediation: a tale of the two worst spills in U.S. history. Environ Sci Technol 45: 6709–6715. doi: 10.1021/es2013227

|
|
[6]
|
Bragg JR, Prince RC, Harner EJ, et al. (1994) Effectiveness of bioremediation for the Exxon Valdez oil spill. Nature 368: 413–418. doi: 10.1038/368413a0

|
|
[7]
|
Day SM (1993) US environmental regulations and policies--their impact on the commercial development of bioremediation. Trends Biotechnol 11: 324–328. doi: 10.1016/0167-7799(93)90154-2

|
|
[8]
|
Caplan JA (1993) The worldwide bioremediation industry: prospects for profit. Trends Biotechnol 11: 320–323. doi: 10.1016/0167-7799(93)90153-Z

|
|
[9]
|
Mishra A, Malik A (2014) Novel fungal consortium for bioremediation of metals and dyes from mixed waste stream. Bioresour Technol 171: 217–226. doi: 10.1016/j.biortech.2014.08.047

|
|
[10]
|
Cerniglia CE (1997) Fungal metabolism of polycyclic aromatic hydrocarbons: past, present and future applications in bioremediation. J Ind Microbiol Biotechnol 19: 324–333. doi: 10.1038/sj.jim.2900459

|
|
[11]
|
Balaji V, Arulazhagan P, Ebenezer P (2014) Enzymatic bioremediation of polyaromatic hydrocarbons by fungal consortia enriched from petroleum contaminated soil and oil seeds. J Environ Biol 35: 521–529.
|
|
[12]
|
Bouwer EJ, Zehnder AJ (1993) Bioremediation of organic compounds--putting microbial metabolism to work. Trends Biotechnol 11: 360–367. doi: 10.1016/0167-7799(93)90159-7

|
|
[13]
|
Bruins MR, Kapil S, Oehme FW (2000) Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45: 198–207. doi: 10.1006/eesa.1999.1860

|
|
[14]
|
Prince RC (2000) Bioremediation. Kirk-Othmer Encyclopedia of Chemical Technology: John Wiley & Sons, Inc.
|
|
[15]
|
Das S, Dash HR (2014) 1 - Microbial Bioremediation: A Potential Tool for Restoration of Contaminated Areas. In: Das S, editor. Microbial Biodegradation and Bioremediation. Oxford: Elsevier. pp. 1–21.
|
|
[16]
|
Joutey NT, Sayel H, Bahafid W, et al. (2015) Mechanisms of hexavalent chromium resistance and removal by microorganisms. Rev Environ Contam Toxicol 233: 45–69.
|
|
[17]
|
Kumar R, Singh S, Singh OV (2007) Bioremediation of radionuclides: emerging technologies. OMICS 11: 295–304. doi: 10.1089/omi.2007.0013

|
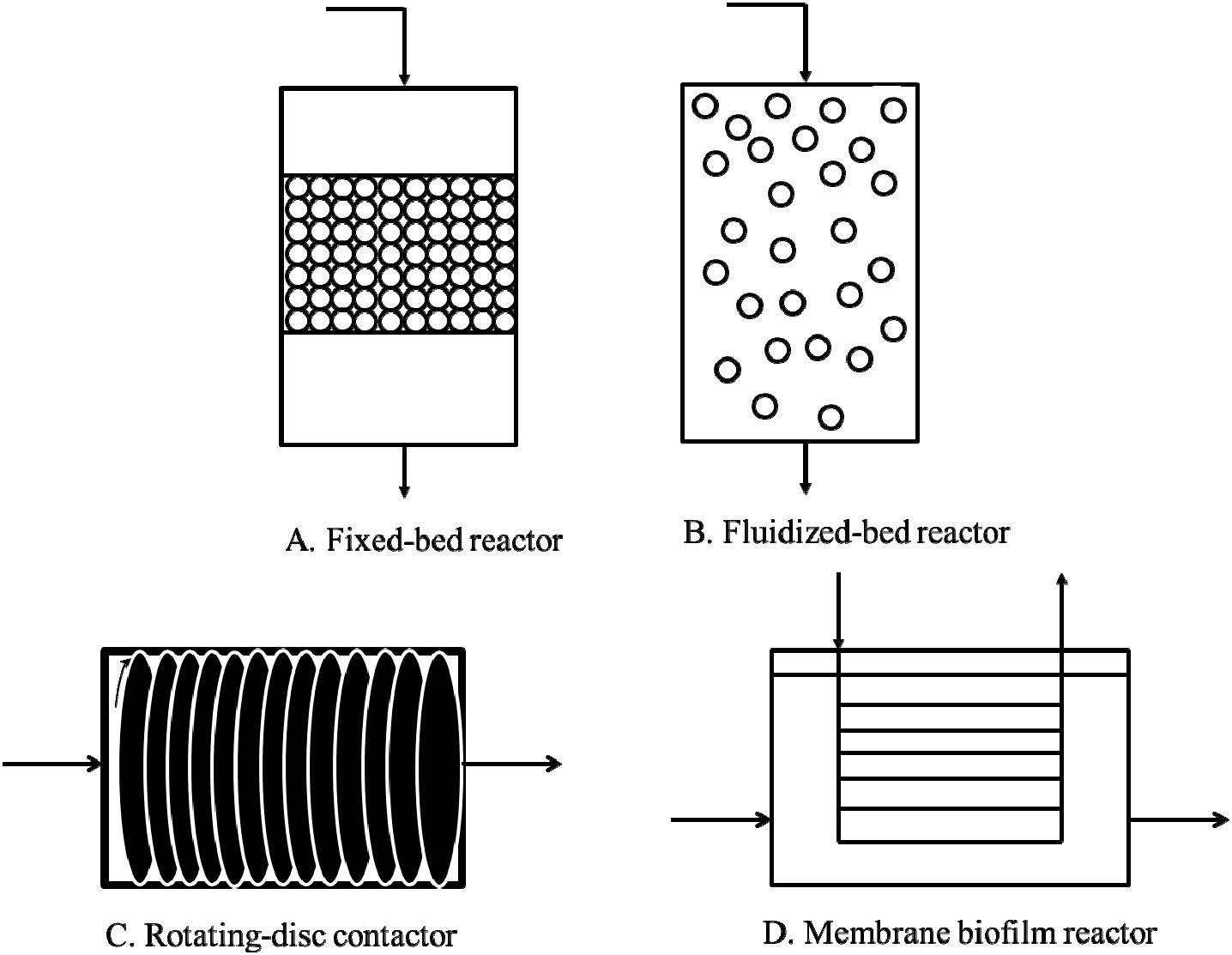
|
[18]
|
Wall JD, Krumholz LR (2006) Uranium reduction. Annu Rev Microbiol 60: 149–166. doi: 10.1146/annurev.micro.59.030804.121357

|
|
[19]
|
Beyenal H, Sani RK, Peyton BM, et al. (2004) Uranium immobilization by sulfate-reducing biofilms. Environ Sci Technol 38: 2067–2074. doi: 10.1021/es0348703

|
|
[20]
|
Vogt C, Richnow HH (2014) Bioremediation via in situ microbial degradation of organic pollutants. Adv Biochem Eng Biotechnol 142: 123–146.
|
|
[21]
|
Jorgensen KS (2007) In situ bioremediation. Adv Appl Microbiol 61: 285–305. doi: 10.1016/S0065-2164(06)61008-3

|
|
[22]
|
Singh JS, Abhilash PC, Singh HB, et al. (2011) Genetically engineered bacteria: an emerging tool for environmental remediation and future research perspectives. Gene 480: 1–9. doi: 10.1016/j.gene.2011.03.001

|
|
[23]
|
Hedlund BP, Staley JT (2001) Vibrio cyclotrophicus sp. nov., a polycyclic aromatic hydrocarbon (PAH)-degrading marine bacterium. Int J Syst Evol Microbiol 51: 61–66.
|
|
[24]
|
Nakajima-Kambe T, Ichihashi F, Matsuzoe R, et al. (2009) Degradation of aliphatic–aromatic copolyesters by bacteria that can degrade aliphatic polyesters. Polym Degrad Stab 94: 1901–1905. doi: 10.1016/j.polymdegradstab.2009.08.006

|
|
[25]
|
Costerton JW, Cheng KJ, Geesey GG, et al. (1987) Bacterial biofilms in nature and disease. Annu Rev Microbiol 41: 435–464. doi: 10.1146/annurev.mi.41.100187.002251

|
|
[26]
|
Gieg LM, Fowler SJ, Berdugo-Clavijo C (2014) Syntrophic biodegradation of hydrocarbon contaminants. Curr Opin Biotechnol 27: 21–29. doi: 10.1016/j.copbio.2013.09.002

|
|
[27]
|
Horemans B, Breugelmans P, Hofkens J, et al. (2013) Environmental dissolved organic matter governs biofilm formation and subsequent linuron degradation activity of a linuron-degrading bacterial consortium. Appl Environ Microbiol 79: 4534–4542. doi: 10.1128/AEM.03730-12

|
|
[28]
|
Pratt LA, Kolter R (1999) Genetic analyses of bacterial biofilm formation. Curr Opin Microbiol 2: 598–603. doi: 10.1016/S1369-5274(99)00028-4

|
|
[29]
|
Lacal J, Reyes-Darias JA, García-Fontana C, et al. (2013) Tactic responses to pollutants and their potential to increase biodegradation efficiency. J Appl Microbiol 114: 923–933. doi: 10.1111/jam.12076

|
|
[30]
|
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8: 623–633.
|
|
[31]
|
More TT, Yadav JSS, Yan S, et al. (2014) Extracellular polymeric substances of bacteria and their potential environmental applications. J Environ Manage 144: 1–25. doi: 10.1016/j.jenvman.2014.05.010

|
|
[32]
|
Flemming HC, Wingender J (2001) Relevance of microbial extracellular polymeric substances (EPSs)--Part II: Technical aspects. Water Sci Technol 43: 9–16.
|
|
[33]
|
Branda SS, Vik S, Friedman L, et al. (2005) Biofilms: the matrix revisited. Trends Microbiol 13: 20–26. doi: 10.1016/j.tim.2004.11.006

|
|
[34]
|
Jung JH, Choi NY, Lee SY (2013) Biofilm formation and exopolysaccharide (EPS) production by Cronobacter sakazakii depending on environmental conditions. Food Microbiol 34: 70–80. doi: 10.1016/j.fm.2012.11.008

|
|
[35]
|
Kreft JU, Wimpenny JW (2001) Effect of EPS on biofilm structure and function as revealed by an individual-based model of biofilm growth. Water Sci Technol 43: 135–141.
|
|
[36]
|
Miqueleto AP, Dolosic CC, Pozzi E, et al. (2010) Influence of carbon sources and C/N ratio on EPS production in anaerobic sequencing batch biofilm reactors for wastewater treatment. Bioresour Technol 101: 1324–1330. doi: 10.1016/j.biortech.2009.09.026

|
|
[37]
|
Reysenbach AL, Cady SL (2001) Microbiology of ancient and modern hydrothermal systems. Trends Microbiol 9: 79–86. doi: 10.1016/S0966-842X(00)01921-1

|
|
[38]
|
Edwards KJ, Bond PL, Gihring TM, et al. (2000) An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science 287: 1796–1799. doi: 10.1126/science.287.5459.1796

|
|
[39]
|
Matz C, Kjelleberg S (2005) Off the hook--how bacteria survive protozoan grazing. Trends Microbiol 13: 302–307. doi: 10.1016/j.tim.2005.05.009

|
|
[40]
|
Davey ME, O'Toole G A (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64: 847–867. doi: 10.1128/MMBR.64.4.847-867.2000

|
|
[41]
|
Mah TF, O'Toole GA (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9: 34–39. doi: 10.1016/S0966-842X(00)01913-2

|
|
[42]
|
Sutherland IW (2001) The biofilm matrix--an immobilized but dynamic microbial environment. Trends Microbiol 9: 222–227. doi: 10.1016/S0966-842X(01)02012-1

|
|
[43]
|
Field JA, Stams AJ, Kato M, et al. (1995) Enhanced biodegradation of aromatic pollutants in cocultures of anaerobic and aerobic bacterial consortia. Antonie Van Leeuwenhoek 67: 47–77. doi: 10.1007/BF00872195

|
|
[44]
|
De Philippis R, Colica G, Micheletti E (2011) Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: molecular basis and practical applicability of the biosorption process. Appl Microbiol Biotechnol 92: 697–708. doi: 10.1007/s00253-011-3601-z

|
|
[45]
|
De Philippis R, Paperi R, Sili C (2007) Heavy metal sorption by released polysaccharides and whole cultures of two exopolysaccharide-producing cyanobacteria. Biodegradation 18: 181–187. doi: 10.1007/s10532-006-9053-y

|
|
[46]
|
Micheletti E, Colica G, Viti C, et al. (2008) Selectivity in the heavy metal removal by exopolysaccharide-producing cyanobacteria. J Appl Microbiol 105: 88–94. doi: 10.1111/j.1365-2672.2008.03728.x

|
|
[47]
|
Iwabuchi N, Sunairi M, Urai M, et al. (2002) Extracellular polysaccharides of Rhodococcus rhodochrous S-2 stimulate the degradation of aromatic components in crude oil by indigenous marine bacteria. Appl Environ Microbiol 68: 2337–2343. doi: 10.1128/AEM.68.5.2337-2343.2002

|
|
[48]
|
Li W-W, Yu H-Q (2014) Insight into the roles of microbial extracellular polymer substances in metal biosorption. Bioresource Technology 160: 15–23. doi: 10.1016/j.biortech.2013.11.074

|
|
[49]
|
Pal A, Paul AK (2008) Microbial extracellular polymeric substances: central elements in heavy metal bioremediation. Indian J Microbiol 48: 49–64. doi: 10.1007/s12088-008-0006-5

|
|
[50]
|
Ferris FG, Schultze S, Witten TC, et al. (1989) Metal Interactions with Microbial Biofilms in Acidic and Neutral pH Environments. Appl Environ Microbiol 55: 1249–1257.
|
|
[51]
|
Zhang HL, Fang W, Wang YP, et al. (2013) Phosphorus removal in an enhanced biological phosphorus removal process: roles of extracellular polymeric substances. Environ Sci Technol 47: 11482–11489. doi: 10.1021/es403227p

|
|
[52]
|
Yuan Z, Pratt S, Batstone DJ (2012) Phosphorus recovery from wastewater through microbial processes. Curr Opin Biotechnol 23: 878–883. doi: 10.1016/j.copbio.2012.08.001

|
|
[53]
|
Burmolle M, Webb JS, Rao D, et al. (2006) Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Appl Environ Microbiol 72: 3916–3923. doi: 10.1128/AEM.03022-05

|
|
[54]
|
Logsdon GS, Kohne R, Abel S, et al. (2002) Slow sand filtration for small water systems. J Environ Eng Sci 1: 339–348. doi: 10.1139/s02-025

|
|
[55]
|
Kartal B, Kuenen JG, van Loosdrecht MC (2010) Engineering. Sewage treatment with anammox. Science 328: 702–703.
|
|
[56]
|
Bengtsson MM, Ovreas L (2010) Planctomycetes dominate biofilms on surfaces of the kelp Laminaria hyperborea. BMC Microbiol 10: 261. doi: 10.1186/1471-2180-10-261

|
|
[57]
|
Fuchs S, Haritopoulou T, Schäfer M, et al. (1997) Heavy metals in freshwater ecosystems introduced by urban rainwater runoff—Monitoring of suspended solids, river sediments and biofilms. Water Sci Technol 36: 277–282.
|
|
[58]
|
Peacock AD, Chang YJ, Istok JD, et al. (2004) Utilization of microbial biofilms as monitors of bioremediation. Microb Ecol 47: 284–292.
|
|
[59]
|
Brummer IH, Fehr W, Wagner-Dobler I (2000) Biofilm community structure in polluted rivers: abundance of dominant phylogenetic groups over a complete annual cycle. Appl Environ Microbiol 66: 3078–3082. doi: 10.1128/AEM.66.7.3078-3082.2000

|
|
[60]
|
Arini A, Feurtet–Mazel A, Maury-Brachet R, et al. (2012) Recovery potential of periphytic biofilms translocated in artificial streams after industrial contamination (Cd and Zn). Ecotoxicology 21: 1403–1414. doi: 10.1007/s10646-012-0894-3

|
|
[61]
|
SzabÓ KÉ, Makk J, Kiss KT, et al. (2008) Sequential colonization by river periphyton analysed by microscopy and molecular fingerprinting. Freshwater Biology 53: 1359–1371. doi: 10.1111/j.1365-2427.2008.01967.x

|
|
[62]
|
Dorigo U, Bérard A, Humbert JF (2002) Comparison of Eukaryotic Phytobenthic Community Composition in a Polluted River by Partial 18S rRNA Gene Cloning and Sequencing. Microbial Ecology 44: 372–380. doi: 10.1007/s00248-002-2024-x

|
|
[63]
|
Kostanjsek R, Lapanje A, Drobne D, et al. (2005) Bacterial community structure analyses to assess pollution of water and sediments in the Lake Shkodra/Skadar, Balkan Peninsula. Environ Sci Pollut Res Int 12: 361–368. doi: 10.1065/espr2005.07.271

|
|
[64]
|
Bricheux G, Le Moal G, Hennequin C, et al. (2013) Characterization and evolution of natural aquatic biofilm communities exposed in vitro to herbicides. Ecotoxicol Environ Saf 88: 126–134. doi: 10.1016/j.ecoenv.2012.11.003

|
|
[65]
|
Ancion PY, Lear G, Dopheide A, et al. (2013) Metal concentrations in stream biofilm and sediments and their potential to explain biofilm microbial community structure. Environ Pollut 173: 117–124. doi: 10.1016/j.envpol.2012.10.012

|
|
[66]
|
Navarro E, Guasch H, Sabater S (2002) Use of microbenthic algal communities in ecotoxicological tests for the assessment of water quality: the Ter river case study. J Appl Phycol 14: 41–48. doi: 10.1023/A:1015242301451

|
|
[67]
|
Dorigo U, Bourrain X, Bérard A, et al. (2004) Seasonal changes in the sensitivity of river microalgae to atrazine and isoproturon along a contamination gradient. Sci Total Environ 318: 101–114. doi: 10.1016/S0048-9697(03)00398-X

|
|
[68]
|
Dewez D, Didur O, Vincent-Héroux J, et al. (2008) Validation of photosynthetic-fluorescence parameters as biomarkers for isoproturon toxic effect on alga Scenedesmus obliquus. Environ Pollution 151: 93–100. doi: 10.1016/j.envpol.2007.03.002

|
|
[69]
|
Schmitt-Jansen M, Altenburger R (2008) Community-level microalgal toxicity assessment by multiwavelength-excitation PAM fluorometry. Aquat Toxicol 86: 49–58. doi: 10.1016/j.aquatox.2007.10.001

|
|
[70]
|
Sayler GS, Layton A, Lajoie C, et al. (1995) Molecular site assessment and process monitoring in bioremediation and natural attenuation. off. Appl Biochem Biotechnol 54: 277–290. doi: 10.1007/BF02787926

|
|
[71]
|
Jorgensen KS, Salminen JM, Bjorklof K (2010) Monitored natural attenuation. Methods Mol Biol 599: 217–233. doi: 10.1007/978-1-60761-439-5_14

|
|
[72]
|
Rittmann BE (2004) Definition, objectives, and evaluation of natural attenuation. Biodegradation 15: 349–357. doi: 10.1023/B:BIOD.0000044587.05189.99

|
|
[73]
|
Tyagi M, da Fonseca MM, de Carvalho CC (2011) Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 22: 231–241. doi: 10.1007/s10532-010-9394-4

|
|
[74]
|
Jansson JK, Bjorklof K, Elvang AM, et al. (2000) Biomarkers for monitoring efficacy of bioremediation by microbial inoculants. Environ Pollut 107: 217–223. doi: 10.1016/S0269-7491(99)00140-2

|
|
[75]
|
Gentry T, Rensing C, Pepper IAN (2004) New Approaches for Bioaugmentation as a Remediation Technology. Crit Rev Environ Sci Technol 34: 447–494. doi: 10.1080/10643380490452362

|
|
[76]
|
Morgan P, Watkinson RJ (1989) Hydrocarbon degradation in soils and methods for soil biotreatment. Crit Rev Biotechnol 8: 305–333. doi: 10.3109/07388558909148196

|
|
[77]
|
Grace Liu P-W, Chang TC, Whang L-M, et al. (2011) Bioremediation of petroleum hydrocarbon contaminated soil: Effects of strategies and microbial community shift. Int Biodeterior Biodegradation 65: 1119–1127. doi: 10.1016/j.ibiod.2011.09.002

|
|
[78]
|
Abeysinghe DH, De Silva DG, Stahl DA, et al. (2002) The effectiveness of bioaugmentation in nitrifying systems stressed by a washout condition and cold temperature. Water Environ Res 74: 187–199. doi: 10.2175/106143002X139901

|
|
[79]
|
Boon N, De Gelder L, Lievens H, et al. (2002) Bioaugmenting bioreactors for the continuous removal of 3-chloroaniline by a slow release approach. Environ Sci Technol 36: 4698–4704. doi: 10.1021/es020076q

|
|
[80]
|
Qureshi N, Annous BA, Ezeji TC, et al. (2005) Biofilm reactors for industrial bioconversion processes: employing potential of enhanced reaction rates. Microb Cell Fact 4: 24. doi: 10.1186/1475-2859-4-24

|
|
[81]
|
Bryers JD (1993) Bacterial biofilms. Curr Opin Biotechnol 4: 197–204. doi: 10.1016/0958-1669(93)90125-G

|
|
[82]
|
Rosche B, Li XZ, Hauer B, et al. (2009) Microbial biofilms: a concept for industrial catalysis? Trends Biotechnol 27: 636–643. doi: 10.1016/j.tibtech.2009.08.001

|
|
[83]
|
Singh R, Paul D, Jain RK (2006) Biofilms: implications in bioremediation. Trends Microbiol 14: 389–397. doi: 10.1016/j.tim.2006.07.001

|
|
[84]
|
Wagner-Dobler I (2003) Pilot plant for bioremediation of mercury-containing industrial wastewater. Appl Microbiol Biotechnol 62: 124–133. doi: 10.1007/s00253-003-1322-7

|
|
[85]
|
Shieh W, Keenan J (1986) Fluidized bed biofilm reactor for wastewater treatment. Bioproducts: Springer Berlin Heidelberg. pp. 131–169.
|
|
[86]
|
Denac M, Dunn IJ (1988) Packed- and fluidized-bed biofilm reactor performance for anaerobic wastewater treatment. Biotechnol Bioeng 32: 159–173. doi: 10.1002/bit.260320206

|
|
[87]
|
Kumar TA, Saravanan S (2009) Treatability studies of textile wastewater on an aerobic fluidized bed biofilm reactor (FABR): a case study. Water Sci Technol 59: 1817–1821. doi: 10.2166/wst.2009.207

|
|
[88]
|
Costley SC, Wallis FM (2001) Bioremediation of heavy metals in a synthetic wastewater using a rotating biological contactor. Water Res 35: 3715–3723. doi: 10.1016/S0043-1354(01)00072-0

|
|
[89]
|
Eker S, Kargi F (2008) Biological treatment of 2,4-dichlorophenol containing synthetic wastewater using a rotating brush biofilm reactor. Bioresour Technol 99: 2319–2325. doi: 10.1016/j.biortech.2007.05.016

|
|
[90]
|
Eker S, Kargi F (2010) COD, para-chlorophenol and toxicity removal from synthetic wastewater using rotating tubes biofilm reactor (RTBR). Bioresour Technol 101: 9020–9024. doi: 10.1016/j.biortech.2010.07.003

|
|
[91]
|
Abraham TE, Senan RC, Shaffiqu TS, et al. (2003) Bioremediation of textile azo dyes by an aerobic bacterial consortium using a rotating biological contactor. Biotechnol Prog 19: 1372–1376.
|
|
[92]
|
Jeswani H, Mukherji S (2012) Degradation of phenolics, nitrogen-heterocyclics and polynuclear aromatic hydrocarbons in a rotating biological contactor. Bioresour Technol 111: 12–20. doi: 10.1016/j.biortech.2012.01.157

|
|
[93]
|
Sarayu K, Sandhya S (2012) Rotating biological contactor reactor with biofilm promoting mats for treatment of benzene and xylene containing wastewater. Appl Biochem Biotechnol 168: 1928–1937. doi: 10.1007/s12010-012-9908-0

|
|
[94]
|
Rittmann BE (2006) The membrane biofilm reactor: the natural partnership of membranes and biofilm. Water Sci Technol 53: 219–225.
|
|
[95]
|
Nerenberg R, Rittmann BE (2004) Hydrogen-based, hollow-fiber membrane biofilm reactor for reduction of perchlorate and other oxidized contaminants. Water Sci Technol 49: 223–230.
|
|
[96]
|
Modin O, Fukushi K, Yamamoto K (2008) Simultaneous removal of nitrate and pesticides from groundwater using a methane-fed membrane biofilm reactor. Water Sci Technol 58: 1273–1279. doi: 10.2166/wst.2008.481

|
|
[97]
|
Fathepure BZ, Vogel TM (1991) Complete degradation of polychlorinated hydrocarbons by a two-stage biofilm reactor. Appl Environ Microbiol 57: 3418–3422.
|
|
[98]
|
Zhang C, Wang L, Yan N, et al. (2013) Air-lift internal loop biofilm reactor for realized simultaneous nitrification and denitrification. Bioprocess Biosyst Eng 36: 597–602. doi: 10.1007/s00449-012-0814-1

|
|
[99]
|
Zhao Y, Feng C, Wang Q, et al. (2011) Nitrate removal from groundwater by cooperating heterotrophic with autotrophic denitrification in a biofilm–electrode reactor. J Hazard Mater 192: 1033–1039. doi: 10.1016/j.jhazmat.2011.06.008

|
|
[100]
|
White C, Gadd GM (1998) Accumulation and effects of cadmium on sulphate-reducing bacterial biofilms. Microbiology 144: 1407–1415. doi: 10.1099/00221287-144-5-1407

|
|
[101]
|
White C, Gadd GM (2000) Copper accumulation by sulfate-reducing bacterial biofilms. FEMS Microbiology Letters 183: 313–318. doi: 10.1111/j.1574-6968.2000.tb08977.x

|
|
[102]
|
Smith WL, Gadd GM (2000) Reduction and precipitation of chromate by mixed culture sulphate-reducing bacterial biofilms. J Appl Microbiol 88: 983–991. doi: 10.1046/j.1365-2672.2000.01066.x

|
|
[103]
|
Hosseini Koupaie E, Alavi Moghaddam MR, Hashemi SH (2013) Evaluation of integrated anaerobic/aerobic fixed-bed sequencing batch biofilm reactor for decolorization and biodegradation of azo dye acid red 18: comparison of using two types of packing media. Bioresour Technol 127: 415–421. doi: 10.1016/j.biortech.2012.10.003

|
|
[104]
|
Lin YH, Hsien TY (2009) Kinetics of biodegradation of phenolic wastewater in a biofilm reactor. Water Sci Technol 59: 1703–1711. doi: 10.2166/wst.2009.203

|
|
[105]
|
Moreno-Andrade I, Buitron G, Vargas A (2009) Effect of starvation and shock loads on the biodegradation of 4-chlorophenol in a discontinuous moving bed biofilm reactor. Appl Biochem Biotechnol 158: 222–230. doi: 10.1007/s12010-008-8392-z

|
|
[106]
|
Coelhoso I, Boaventura R, Rodrigues A (1992) Biofilm reactors: an experimental and modeling study of wastewater denitrification in fluidized-bed reactors of activated carbon particles. Biotechnol Bioeng 40: 625–633. doi: 10.1002/bit.260400510

|
|
[107]
|
Masic A, Eberl HJ (2014) A modeling and simulation study of the role of suspended microbial populations in nitrification in a biofilm reactor. Bull Math Biol 76: 27–58. doi: 10.1007/s11538-013-9898-2

|
|
[108]
|
Martin KJ, Picioreanu C, Nerenberg R (2015) Assessing microbial competition in a hydrogen-based membrane biofilm reactor (MBfR) using multidimensional modeling. Biotechnol Bioeng.
|
|
[109]
|
Valls M, de Lorenzo Vc (2002) Exploiting the genetic and biochemical capacities of bacteria for the remediation of heavy metal pollution. FEMS Microbiol Rev 26: 327–338. doi: 10.1111/j.1574-6976.2002.tb00618.x

|
|
[110]
|
Diels L, De Smet M, Hooyberghs L, et al. (1999) Heavy metals bioremediation of soil. Molecular Biotechnology 12: 149–158. doi: 10.1385/MB:12:2:149

|
|
[111]
|
Macaskie LE, Yong P, Doyle TC, et al. (1997) Bioremediation of uranium-bearing wastewater: biochemical and chemical factors influencing bioprocess application. Biotechnol Bioeng 53: 100–109.
|
|
[112]
|
Shukla SK, Mangwani N, Rao TS, et al. (2014) 8 - Biofilm-Mediated Bioremediation of Polycyclic Aromatic Hydrocarbons. In: Das S, editor. Microbial Biodegradation and Bioremediation. Oxford: Elsevier. pp. 203–232.
|
|
[113]
|
Chen M, Xu P, Zeng G, et al. (2015) Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol Adv 33: 745–755.
|
|
[114]
|
Cerniglia C (1993) Biodegradation of polycyclic aromatic hydrocarbons. In: Rosenberg E, editor. Microorganisms to Combat Pollution: Springer Netherlands. pp. 227–244.
|
|
[115]
|
Jones KC, de Voogt P (1999) Persistent organic pollutants (POPs): state of the science. Environ Pollut 100: 209–221. doi: 10.1016/S0269-7491(99)00098-6

|
|
[116]
|
Bonefeld-Jorgensen EC, Hjelmborg PS, Reinert TS, et al. (2006) Xenoestrogenic activity in blood of European and Inuit populations. Environ Health 5: 12. doi: 10.1186/1476-069X-5-12

|
|
[117]
|
Johnsen AR, Karlson U (2004) Evaluation of bacterial strategies to promote the bioavailability of polycyclic aromatic hydrocarbons. Appl Microbiol Biotechnol 63: 452–459.
|
|
[118]
|
Rodriguez S, Bishop P (2008) Enhancing the Biodegradation of Polycyclic Aromatic Hydrocarbons: Effects of Nonionic Surfactant Addition on Biofilm Function and Structure. J Environ Eng 134: 505–512. doi: 10.1061/(ASCE)0733-9372(2008)134:7(505)

|
|
[119]
|
Plosz BG, Vogelsang C, Macrae K, et al. (2010) The BIOZO process--a biofilm system combined with ozonation: occurrence of xenobiotic organic micro-pollutants in and removal of polycyclic aromatic hydrocarbons and nitrogen from landfill leachate. Water Sci Technol 61: 3188–3197. doi: 10.2166/wst.2010.920

|
|
[120]
|
Song HG, Bartha R (1990) Effects of jet fuel spills on the microbial community of soil. Appl Environ Microbiol 56: 646–651.
|
|
[121]
|
Ron EZ, Rosenberg E (2014) Enhanced bioremediation of oil spills in the sea. Curr Opin Biotechnol 27: 191–194. doi: 10.1016/j.copbio.2014.02.004

|
|
[122]
|
Harayama S, Kasai Y, Hara A (2004) Microbial communities in oil-contaminated seawater. Curr Opin Biotechnol 15: 205–214. doi: 10.1016/j.copbio.2004.04.002

|
|
[123]
|
Dasgupta D, Ghosh R, Sengupta TK (2013) Biofilm-mediated enhanced crude oil degradation by newly isolated pseudomonas species. ISRN Biotechnol 2013: 250749.
|
|
[124]
|
Mnif I, Mnif S, Sahnoun R, et al. (2015) Biodegradation of diesel oil by a novel microbial consortium: comparison between co-inoculation with biosurfactant-producing strain and exogenously added biosurfactants. Environ Sci Pollut Res Int 22: 14852–14861. doi: 10.1007/s11356-015-4488-5

|
|
[125]
|
Koren O, Knezevic V, Ron EZ, et al. (2003) Petroleum pollution bioremediation using water-insoluble uric acid as the nitrogen source. Appl Environ Microbiol 69: 6337–6339. doi: 10.1128/AEM.69.10.6337-6339.2003

|
|
[126]
|
Muyzer G, Stams AJ (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol 6: 441–454.
|
|
[127]
|
Erable B, Duţeanu NM, Ghangrekar MM, et al. (2009) Application of electro-active biofilms. Biofouling 26: 57–71.
|
|
[128]
|
Li Z, Zhang X, Lei L (2008) Electricity production during the treatment of real electroplating wastewater containing Cr6+ using microbial fuel cell. Process Biochemistry 43: 1352–1358. doi: 10.1016/j.procbio.2008.08.005

|
|
[129]
|
Cong Y, Xu Q, Feng H, et al. (2013) Efficient electrochemically active biofilm denitrification and bacteria consortium analysis. Bioresour Technol 132: 24–27. doi: 10.1016/j.biortech.2013.01.004

|
|
[130]
|
Heitzer A, Sayler GS (1993) Monitoring the efficacy of bioremediation. Trends Biotechnol 11: 334–343. doi: 10.1016/0167-7799(93)90156-4

|
|
[131]
|
Perumbakkam S, Hess TF, Crawford RL (2006) A bioremediation approach using natural transformation in pure-culture and mixed-population biofilms. Biodegradation 17: 545–557. doi: 10.1007/s10532-005-9025-7

|
|
[132]
|
Urgun-Demirtas M, Stark B, Pagilla K (2006) Use of Genetically Engineered Microorganisms (GEMs) for the Bioremediation of Contaminants. Crit Rev Biotechnol 26: 145–164. doi: 10.1080/07388550600842794

|
|
[133]
|
Cases I, de Lorenzo V (2005) Genetically modified organisms for the environment: stories of success and failure and what we have learned from them. Int Microbiol 8: 213–222.
|
|
[134]
|
Absalon C, Ymele-Leki P, Watnick PI (2012) The bacterial biofilm matrix as a platform for protein delivery. MBio 3: e00127–00112.
|
|
[135]
|
Safa M, Alemzadeh I, Vossoughi M (2014) Biodegradability of oily wastewater using rotating biological contactor combined with an external membrane. J Environ Health Sci Eng 12: 117. doi: 10.1186/s40201-014-0117-3

|
|
[136]
|
Kaindl N (2010) Upgrading of an activated sludge wastewater treatment plant by adding a moving bed biofilm reactor as pre-treatment and ozonation followed by biofiltration for enhanced COD reduction: design and operation experience. Water Sci Technol 62: 2710–2719. doi: 10.2166/wst.2010.938

|









 DownLoad:
DownLoad: