1. Introduction
In recent years, naturally ventilated buildings have been replaced by more-energy-efficient airtight buildings to improve the energy efficiency for air conditioning. Consequently, many types of air-polluting chemicals from artificial building materials accumulate in the indoor environment [1,2,3], causing health concerns such as the “sick-building syndrome” [4,5].
Acetaldehyde is one of the major indoor pollutants in Japan, and it is emitted from furniture fabricated from particleboards and treated with urea-formaldehyde resins [6,7]. Because acetaldehyde has direct mutagenic and carcinogenic effects [8,9], the guideline value for the indoor air concentration of acetaldehyde was established as 30 ppb (parts per billion by volume) by the Ministry of Health, Labour and Welfare, Japan [10]. The hydrophilic nature of acetaldehyde results in little removal by activated carbon absorption, which is the most common air-cleaning method.
The wet scrubbing technique, in which hydrophilic air pollutants are removed by mass transfer from the gas phase into the liquid phase, is a promising method for removing hydrophilic air pollutants from the gas phase [11]. The mass transfer rate depends on the concentration difference between the gas phase and the liquid phase. A higher concentration difference leads to a higher mass transfer rate. The increase in the concentration of a pollutant in the liquid phase as a consequence of gas absorption reduces the efficiency of removal of the air pollutant. Thus, re-emission of absorbed air pollutants may occur when the pollutant concentration in the gas phase is lower than that in liquid phase. Furthermore, hydrophobic air pollutants are not removed by the wet scrubbing process due to their low solubility in water. These limitations of the wet scrubbing process have been addressed by combination with other techniques such as photocatalytic degradation and advanced oxidation processes [11,12].
Among the reported combined wet scrubbing processes, the combination of the wet scrubbing process with the photo-Fenton reaction has been particularly attractive as a promising air-cleaning process [13,14,15]. The removal of an air pollutant from the gas phase by means of this combined process involves mass transfer from the gas phase into the liquid phase with simultaneous chemical reaction in the liquid phase. In this combined process, an air pollutant absorbed into the liquid phase is degraded by hydroxyl (OH) radicals produced by the photo-Fenton reaction, as shown in Equations 1 and 2 [16]. The air pollutant is then ultimately mineralized to carbon dioxide and water[17].
|
Fe2+ + H2O2 → Fe3+ + ⋅OH + OH−
|
(1) |
|
Fe3+ + H2O + hv → Fe2+ + ⋅OH + H+
|
(2) |
Because an air pollutant that is transferred from the gas phase into the liquid phase is degraded by the degradation reaction, the concentration of this pollutant in the liquid phase remains low [14]; therefore, high removal efficiency may be maintained due to the occurrence of the photo-Fenton reaction in the liquid phase.
In our previous paper, the removal of gaseous toluene, as a hydrophobic gas, by combination of the wet scrubbing process with the photo-Fenton reaction was investigated [14]. Although the removal of acetaldehyde, as a hydrophilic gas, by the proposed process was briefly investigated in our previous paper [15], in this study, more detailed study to clear the mechanisms of the proposed process was carried out. The effects of the experimental parameters (initial total-iron-ion concentration, initial hydrogen peroxide, and UV light irradiation) on the removal efficiency, which is defined as the ratio of the concentration of removed acetaldehyde-gas to the inlet acetaldehyde-gas concentration, were examined.
2. Materials and Method
2.1. Chemicals
Reagent grade hydrogen peroxide (30% solution) was obtained from Kanto Chemical Co., Inc. (Japan). Iron(II) sulfate heptahydrate (FeSO4⋅7H2O) and sulfuric acid were purchased from Wako Pure Chemical Industries (Japan) and used without further purification.
2.2. Method
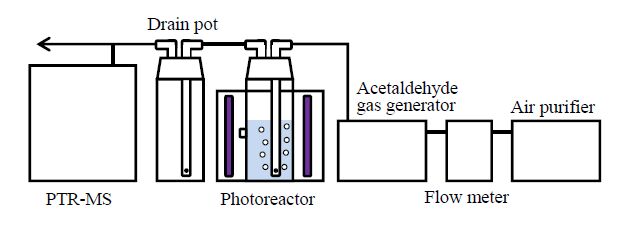
The experimental setup is shown in Figure1. Experiments were carried out in a semi-batch system consisting of a Pyrex glass reactor with a diameter, height, and working volume of 5.5 cm, 20cm, and 0.2 L, respectively. Acetaldehyde-gas was generated by a gas generator (PD-1B, GASTEC, Japan) equipped with a permeation tube (P-92-1, GASTEC, Japan). The inlet acetaldehyde-gas concentration was 1000 ppb, and the gas flow rate was 700 mL min−1. The pH of the reaction solution was adjusted to 3.0 (using sulfuric acid) which is the optimum pH for the photo-Fenton reaction [18]; no pH buffers were used in any of the experiments. The UV irradiation source consisted of three 4 W near-UV (black light) fluorescent lamps (SLG-4WNA, Nihon Global Shomei, Japan) that had a radiation peak at 352 nm. The lamps were located around the cylindrical photoreactor and were used for external irradiation of the solution. The distance between the photoreactor wall and the lamps was 3 cm. The total UV light intensity at the surface of the photo reactor was measured with a UV radiometer (UVR-300, Topcon, Japan), and it was found to be 62.3W m−2.
All experiments were initiated by the addition of a known amount of hydrogen peroxide to the solution containing iron(II) sulfate heptahydrate (as a source of iron), followed by injection of acetaldehyde-gas into the solution, with subsequent irradiation using the UV lamps. Samples from the liquid phase were withdrawn at predetermined time intervals using a syringe. The concentration of hydrogen peroxide in the sample solutions was measured using a method adopted from the glucose oxidase method [19]. The total-iron-ion (ferrous and ferric ions) concentration was measured using the 1, 10-phenanthroline method (Shibata Sci. Technol., Japan) [20].
During the experiments, samples from the gas phase were withdrawn continuously and the concentrations of organic gases, including incompletely oxidized intermediates, were analyzed by means of PTR-MS (Proton Transfer Reaction Mass Spectrometry) (PTR-QMS 500, Ionicon Analytik GmbH., Austria). The mass range from m/z 22 to 95 was analyzed in this study. PTR-MS is a quadrupole mass spectrometer that uses hydronium ions (H3O+) to chemically ionize the compound of interest through a proton transfer reaction. Thus, any compound that has a proton affinity higher than that of water can be detected and identified based on its molecular weight plus 1 (H+). The PTR-MS technique can be used to detect highly polar molecules such as oxidized organic compounds, and the short accumulation time (50 ms per mass number) of the method enables real-time measurements [21,22].
3. Results and Discussion
3.1. Removal of acetaldehyde from the gas phase by the photo-Fenton reaction
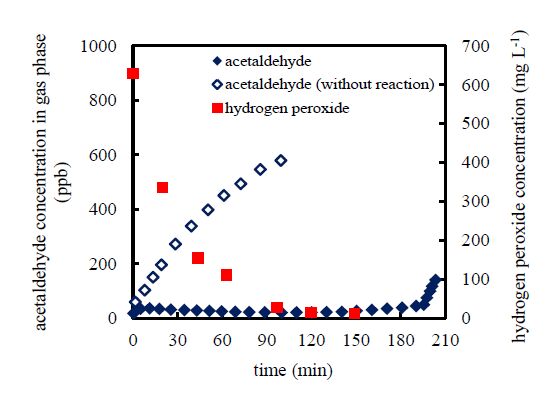
The efficacy of the photo-Fenton reaction for the removal of acetaldehyde-gas from the liquid phase was evaluated by comparison of the temporal variation of the outlet acetaldehyde-gas concentration in the presence and absence of the photo-Fenton reaction. Typical data (acquired under the following conditions: inlet acetaldehyde-gas concentration of 1000 ppb, initial total-iron-ion concentration of 20 mg L−1, initial hydrogen peroxide concentration of 630 mg L−1, and initial solution pH of 3; under UV light irradiation) are depicted in Figure2.
The data presented in Figure2 demonstrate that the outlet acetaldehyde-gas concentration remained at 20 ppb while hydrogen peroxide was present in the solution (<150 min). This implies that around 98% of the injected acetaldehyde-gas was dissolved in the liquid phase and was continuously removed until the photo-Fenton reaction was completed in the liquid phase. The decline in the hydrogen peroxide concentration is indicative of its consumption by the photo-Fenton reaction. Hydrogen peroxide is a key reagent in the photo-Fenton reaction, as expressed in Equation 1. Therefore, complete consumption of hydrogen peroxide quenches the photo-Fenton reaction. As the concentration of hydrogen peroxide declines, the photo-Fenton reaction is gradually depressed, and the outlet acetaldehyde-gas concentration is observed to increase gradually with time. Following this gradual increase, there is a drastic increase in the outlet acetaldehyde-gas concentration at 197 min. Even after the exhaustion of the hydrogen peroxide, the outlet acetaldehyde-gas concentration remained low. This may be attributed to the effects of incompletely oxidized intermediates in that certain incompletely oxidized intermediates may react directly with acetaldehyde and/or with ferrous and ferric ions, leading to continuation of the iron-redox cycle to produce hydroxyl radicals [23,24].
In the absence of the photo-Fenton reaction, the acetaldehyde-gas could still be removed (the outlet acetaldehyde-gas concentration at 1.6 min was 59 ppb) due to the absorption of the acetaldehyde-gas into the liquid phase. Because acetaldehyde is a hydrophilic gas, it dissolves readily in water. However, the absorption of acetaldehyde-gas into the aqueous system has a saturation limit. Moreover, increasing the acetaldehyde concentration in the liquid phase depresses the absorption rate. Consequently, the outlet acetaldehyde-gas concentration increased with time in the absence of the photo-Fenton reaction.
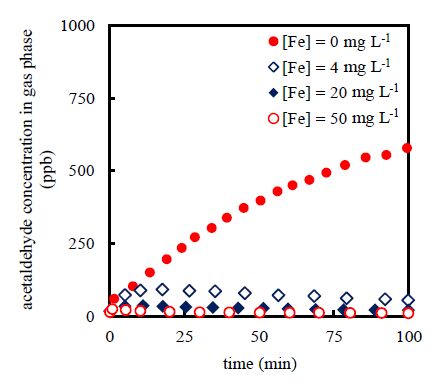
3.2. Effects of initial total-iron-ion concentration
In general, the hydroxyl-radical-generation performance of the photo-Fenton reaction is mainly affected by the initial total-iron-ion concentration in the presence of an adequate amount of hydrogen peroxide. In order to estimate the effects of the initial total-iron-ion concentration on the air-cleaning performance, the initial total-iron-ion concentration was varied from 0 to 50 mg L−1 for the fixed inlet acetaldehyde-gas concentration of 1000 ppb using an initial hydrogen peroxide concentration of 630 mg L−1. Figure3 shows the temporal variation in the outlet acetaldehyde-gas concentration at different initial total-iron-ion concentrations. In the absence of the photo-Fenton reaction in the liquid phase ([Fe] = 0 mg L−1; hydrogen peroxide solution, Figure3: closed circles), the outlet acetaldehyde-gas concentration increased with time. However, in the cases in which the photo-Fenton reaction occurred in the liquid phase ([Fe] = 4 to 50 mg L−1, Figure3: open circles and diamonds), the outlet acetaldehyde-gas concentration did not increase with time and achieved a steady state. A higher initial total-iron-ion concentration resulted in a lower outlet acetaldehyde-gas concentration. At [Fe] = 50 mg L−1, more than 99% of the inlet acetaldehyde-gas could be removed. Even when a low initial total-iron-ion concentration ([Fe] = 4 mg L−1) was used, more than 95% of the inlet acetaldehyde-gas could be removed. Increasing the initial total-iron-ion concentration enhanced the photo-Fenton reaction and decreased the acetaldehyde concentration in the liquid phase due to the degradation of acetaldehyde by hydroxyl radicals, thereby improving the mass transfer of acetaldehyde from the gas phase into the liquid phase. Consequently, the outlet acetaldehyde-gas concentration at the steady state decreased with an increase in the initial total-iron-ion concentration.
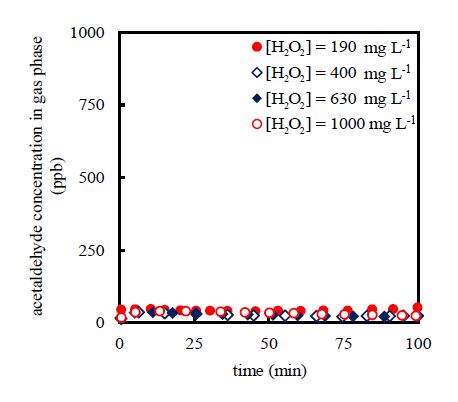
3.3. Effects of initial hydrogen peroxide concentration
The effects of the initial hydrogen peroxide concentration on the acetaldehyde-gas removal wereevaluated by varying the initial hydrogen peroxide concentration from 190 to 1000 mg L−1 for the fixed inlet acetaldehyde-gas concentration of 1000 ppb using an initial total-iron-ion concentration of 20 mg L−1. The experimental results are shown in Figure4. For all of the initial hydrogen peroxide concentrations evaluated, the same trend was observed, and the outlet acetaldehyde-gas concentrations fell within the range of 20-47 ppb in all cases. In general, a higher initial hydrogen peroxide concentration improved the formation of hydroxyl radicals. Excess hydrogen peroxide also enhances the formation of hydroxyl radicals. Nevertheless, in the presence of large quantities of hydroxyl radicals, there is reaction of hydroxyl radicals with other hydroxyl radicals, and consequently, the degradation rate of dissolved acetaldehyde was not affected. In other words, hydrogen peroxide acted as a scavenger of the highly reactive hydroxyl radicals to form hydroperoxyl radicals. For this reason, the initial hydrogen peroxide concentration did not exert a significant influence on the outlet acetaldehyde-gas concentration within the range of initial concentrations evaluated.
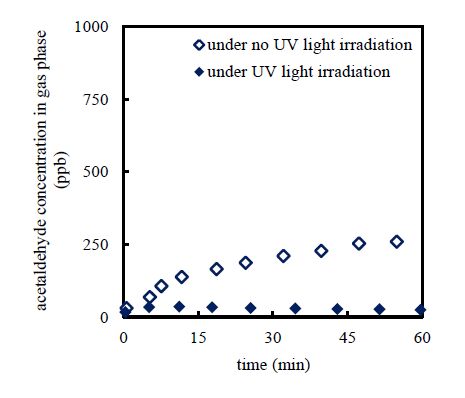
3.4. Effects of UV light irradiation
The effects of UV light irradiation on the acetaldehyde-gas removal are illustrated in Figure5. Experiments were performed using an inlet acetaldehyde-gas concentration of 1000 ppb, initial total-iron-ion concentration of 20 mg L−1, and an initial hydrogen peroxide concentration of 630mgL−1 in the absence and presence of UV irradiation. The acetaldehyde-gas removal was enhanced in presence of UV irradiation. The photosensitive ferric ion is reduced to the ferrous ion under UV light irradiation (Equation 2). The Fenton reaction (Equation 1) is enhanced by the presence of photogenerated ferrous ions. Consequently, the removal of acetaldehyde-gas was enhanced under UV irradiation.
4. Conclusion
The data presented herein suggest that the air-cleaning protocol combining the wet scrubbing process with the photo-Fenton reaction is very effective for the removal of air pollutants from an indoor environment. 99% removal efficiency was achieved in the one-pass test (residence time of 17s) using acetaldehyde-gas at an inlet concentration of 1000 ppb as the analyte, at an initial total-iron-ion concentration of 50 mg L−1 and initial hydrogen peroxide concentration of 630 mg L−1. Even at the low initial total-iron-ion concentration of 4 mg L−1, a removal efficiency of 92% was achieved, and this value was further improved by increasing the initial total-iron-ion concentration. The acetaldehyde removal efficiency was relatively independent of the initial hydrogen peroxide concentration. UV irradiation further augmented the rate of the photo-Fenton reaction leading to enhanced acetaldehyde-gas removal.
Conflict of interest
All authors declare no conflicts of interest in this paper.









 DownLoad:
DownLoad: