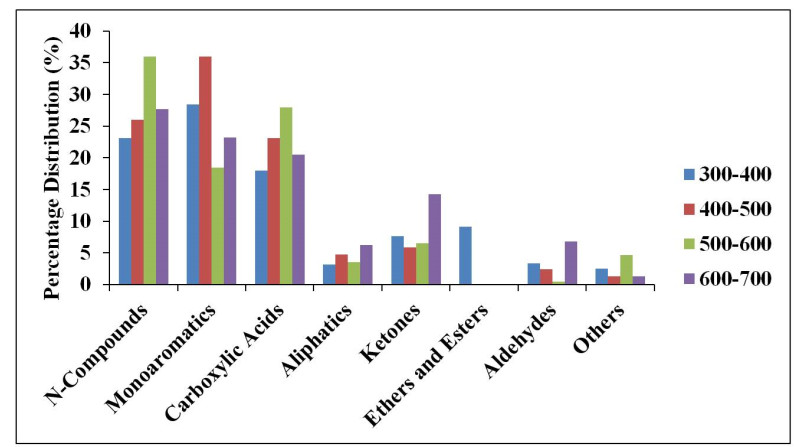
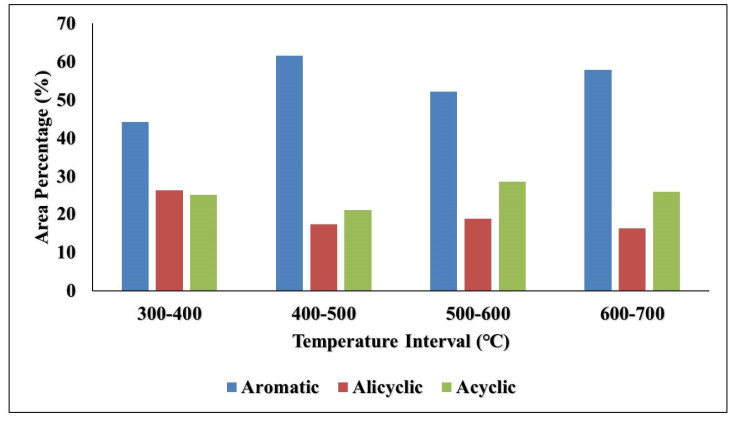
Sewage sludge is a very harmful waste when improperly discharged into the environment because of its inherent abundant pathogens, organic pollutants, and heavy metal constituents. The pyrolysis of sewage sludge is viewed not only to reduce pollutants associated with it but also one of the viable alternative sources for renewable energy or biofuel production. In this study, the effect of catalyst and temperature on the yield and composition of bio-oil obtained from the catalytic and non-catalytic pyrolysis of desludging sewage samples (DSS) was investigated. Modified pyrolysis reactor was used to pyrolyze the DSS at temperature ranges of 300–400,400–500,500–600 and 600–700 ℃ with and without the use of zeolite-Y catalyst. The 'heterogeneous' catalysis reaction yielded 20.9 wt% bio-oil, while the reaction without catalyst yielded 18.2 wt% bio-oil. Pyrolysis of the DSS favored char yield of between 55.4 and 76.6 wt%. The X-ray Fluorescence (XRF) analysis showed high silica (46 and 56.1 wt%), calcium (20.9 and 15.50 wt%), and low organic matter (12 and 12.87 wt%) contents present in the desludging feedstock before and after pyrolysis respectively. The gas chromatography–mass spectrometry (GCMS) analysis indicated the presence of nitrogen-containing compounds (between 20 and 50 wt%), mono-aromatics (18 and 28 wt%) and oxygenated compounds, in the form of carboxylic acids, aliphatics, ketones, ethers, esters and aldehydes in the bio-oils. Pyrolysis process development is, therefore, essential to clean the environment of pollutants from sewage sludge, by its conversion to more useful chemicals. In contrast, sewage sludge with high silica content may be tailored to the production of building materials.
Abbreviations: GAE/g: Gallic acid equivalents per one gram; CE/g: Catechin equivalents (CE) per one gram; RE/g: Rutin equivalents per one gram; QuE: Quercetin equivalent
1.
Introduction
Pomegranate is native to Persia and Mediterranean zone and has been widely used in many countries and cultures [1]. Pomegranate has attracted considerable attention for its health benefits in recent years. Results show that pomegranate juice has markedly high total phenolic contents and antioxidant capacity, being responsible for beneficial activities of pomegranates. Total phenolic content plays a probable role in preventing different diseases related to oxidative stress such as cardiovascular [2,3,4], cancer [5,6] and neurodegenerative diseases [7,8]. Pomegranate juice has excellent antioxidant activity and is beneficial for atherosclerosis prevention [2,3,4], In this regard, polyphenols are capable of moderating the broad range of enzymes activities and cell receptors [9]. The main part of pomegranate seed oil is Punicic acid having anti-atherogenic effects [10].
Pomegranate extract has potential to decrease the incidence of collagen-induced arthritis. In an in-vivo study into mice fed by pomegranate extract, it was indicated that joint inflammation, arthritis severity and IL-6 level were decreased remarkably [11]. Pomegranate has been widely accepted for its antimicrobial [12,13,14], and anti-candidial activities by in-vitro [15] and iv-vivo investigations [16]. Crude extract of pomegranate peel yielded a compound that demonstrated potent antifungal activity to Candida spp. [13]. The compound was recognized as punicalagin, based on spectral analyses [17]. Punicalagin showed high activity against Candida albicans and Candida parapsilosis, as tested by minimum inhibitory concentration (MIC) [14]. Pomegranate pericarp and peel extracts are reported to possess strong activity against Candida spp., with MICs of 125 μg/mL [18]. Salazar Aranda et al. [19] showed that polyphenolics compound had antioxidant activity, and they revealed its highest activity against C. glabrata by MIC test. The effect of their hydroxyl groups on their activity against C. glabrata is considerable. Furthermore, it has been shown that pomegranate peel has a high inhibition capacity against C. albicans [19,20]. Bassiri-Jahromi et al. in their in-vitro [15] and in-vivo [16] investigations indicated that pomegranate peel extract had potential antifungal activity against 5 various Candida species. This investigation demonstrated that among 8 different Persian pomegranate cultivars, Saveh sour malas peel extract indicated strongest antifungal activity against C.albicans, which was comparable to nystatin. Bassiri-Jahromi et al. reported that pomegranate peel extract had no adverse effects following application in the rats' model. Pomegranate peel extract application was effective and safe in treating oral candidiasis in the Wistar rats [21].
Schubert et al. (1999) in their studies clearly demonstrated that pomegranate fermented juice and seed procurement and pomegranate seed oil contained powerful antioxidant properties [22]. Therefore, pomegranate can play a potential role as natural food preservative, health promotion and therapeutic agents.
This paper provides a general review of the evaluation of polyphenolic and flavonoid contents of various pomegranate cultivars in different regions of the world.
2.
Pomegranate polyphenols and flavonoid compound
Pomegranate has considerable content of phytochemicals compounds such as punicalagin, ellagitannins, anthocyanins, tannins, hydrolysable tannins, and punicic acid [18,19,20,21,22,23]. Pomegranate peel is a valuable source of polyphenolic compounds, known as punicalagin, which is an ellagitannin with antioxidant capacity and is unrivaled and unique to pomegranate [24].
Phenolic compounds have attracted increasing attention as agents for inhibiting and treating various oxidative stress correlated diseases, preventing conventional and novel biomarkers of tissue plasminogen activator (TPA) induced tumor promotion, as well as possessing chemo-preventive role in various tumor models [25]. These compounds are recognized for their attributes in scavenging free radicals and preventing in-vitro lipid oxidation [26,27].
Polyphenol is a significant antioxidant found in pomegranate seed and juice containing ellagitannin (punicalagin), gallic acid, ellagic acid, anthocyanins, catechins, caffeic acid, and quercetin [28]. Flavonoids may prevent coronary artery diseases by inducing various processes such as HDL increase, LDL decrease level, mast cell release reduction, and cardiovascular inflammation decrease. Flavonoids have been recognized with antiviral activity since the 1940s [29]. Selway et al. (1986) have reported that flavonoids contain antiviral activity against 11 types of viruses [30]. Furthermore, flavonoids possess protective effect against liver injury [31]. There is incisive documentary evidence that flavonoids have anti-mutagenic acting [32,33].
Pomegranate peel is a significant source of bioactive compounds such as flavonoids, polyphenols, ellagitannins, and proanthocyanidin [34]; however, this part of the fruit is inedible. The antibacterial activity of peel extracts of Indian Ganesh variety was tested by Malviya et al. using the agar well diffusion method against four bacterial strains, Staphylococcus aureus, Salmonella typhi, Enterobacter aerogenes, and Klebsiella pneumoniae. The pomegranate peel extracts showed significant antibacterial activities against all of the 4 bacterial strains tested [35].
The abundance of these compounds and their activities are related to cultivar type, climate, and growing region [36,37]. Up to now, polyphenols of different pomegranate cultivars in Iran [38], Turkey [39], the United States [40], Italy [41] and South Africa have been investigated [42]. Fawole et al. showed that the highest peel extract activity against monophenolase activity and phenolase activity was Bhagwa cultivar and Arakta cultivar with IC50 values of 3.66 μg/mL and 15.88 μg/mL, respectively [43].
Almost 50% of the pomegranate weight corresponds to the peel [44]. Total polyphenols, flavonoids and pro-athocyanidins contents are superior in pomegranate peel extract than in pomegranate pulp extract owing to their powerful antioxidant capacity [45].
According to Shams Ardekani et al. (2011) [38] report from Iran, sour summer cultivar peel extract has the highest antioxidant activity with 118.074 mg or 274.132 μ mol trolox/g. Sour summer cultivar is a strong source of natural antioxidants, phenolic and flavonoid content and the peel of Sweet Saveh malas, Sour summer and Black peel cultivars are suitable sources of phenolic and flavonoid compounds.
This review will investigate the evidence for the identity of the antioxidant content of various cultivars of pomegranate such as polyphenols and flavonoids, playing a probable role in preventing different diseases associated with oxidative stress such as cardiovascular, cancer and neurodegenerative diseases.
The results provide significant information about the compound of polyphenols and antioxidant content of different cultivars of pomegranate, which can be useful for expanding fruit processing professions and choosing favorable pomegranate genotypes to provide commercial agriculture.
The phenolic and flavonoids content are different; the antioxidant activity of various solvent extracts from pomegranate peel was also surveyed using in vitro assays.
Gil et al. (2000) [27] evaluated antioxidant acting of pomegranate by four comparative assays: ABTS, DPPH, DMPD, FRAP, and they were detected and quantified using ellagic acid anthocyanins, and hydrolyzable tannins in pomegranate juice. They reported that the antioxidant capacity of commercial pomegranate juice was three times superior to red wine and green tea.
Pomegranate peels have significant superior antioxidant potency compared to other parts of pomegranate against free radical activities. It also contains higher total polyphenols, flavonoids and pro-athocyanidins than pulp extract. Strong antioxidant potency of pomegranate peel extract may be due to its major polyphenolics contained [46]. In the present study, various cultivars from different regions were described evaluate the phenolic and flavonoid contents. Tables 1, 2 and 3 present the obtained data.
3.
Discussion
Owing to the pomegranate health benefits, consumption of fresh pomegranates juice is increasing. Rich bioactive compound cultivars are a significant source of desirable antioxidant properties for health promotion. Pomegranate peel and pulp contain various kinds of antioxidants; however, pomegranate peel had the most antioxidant efficacy compared to the pulp and seed fractions [47].
The results of a number of investigations on phenolic compounds and antioxidant capacity of eighteen various pomegranate cultivars grown in Morocco revealed that thepolyphenols concentration in pomegranate was high, and antioxidant activity and physico-chemical characteristics in pomegranates were influenced by the type of cultivar. Phenolic compounds of pomegranates are graded on phenolic acids (ellagic acid, gallic acid, chlorogenic acid, caffeic acid, vanillic acid, ferulic acids trans-2- Hydrocinnamic acid, quercetin). Additionally, some flavonoids such as catechin, rutin, quercetin and phloridzin were identified in pomegranate juice at various concentrations among the pomegranate cultivars [48].
Although pomegranate peels and the other remaining tissues are inedible, it would be possible to use them to prepare new products such as flavonoids capsules and other nutraceuticals after extraction. There are many pomegranate cultivars, which are classified and correlated based on some important parameters such as morphological characteristics of flower and tree.
Differences in the phenolic compound index among various parts of pomegranate were observed. Pomegranate peels indicated a high concentration of phenolic compounds, and ellagitannins have largest quantities in relation to pomegranate pulp and juice for each cultivar [49]. Owing to meeting the current market demand for fruits quality, the characteristics of pomegranate cultivars are important.
This paper provides an overview of biology evaluation of the phenolic and flavonoid contents of different pomegranate cultivars.
This review described a polyphenolic and flavonoid analysis and geographic origins of different pomegranate cultivars.
Different parts of the pomegranate such as peel can act as potentially antimicrobial agents. Table 1 shows that the Ganesh cultivar possesses the highest polyphenilic compound, indicating an association between polyphenols level and antibacterial activities. Bassiri-Jahromi et al. in their in-vivo investigation indicated that Saveh sour malas Persian cultivar possessed the best activity against 8 Candida strains [16]. Owing to the significant amount of phenolic compounds, Saveh sour malas is one of the best cultivars (Table 1). These findings demonstrated the relationship between the amount of polyphenolic compounds and its anti-candidiasis effect.
Therefore, it may be suggested as a natural alternative to synthetic antimicrobial agents. Punicalagin content in pomegranate extract is tannin, which is reported to be responsible for antimicrobial activity. Furthermore, the tannin rich bioactive fractions and ellagitannins have antibacterial [50], antifungal [51] and antimalaria properties [52].
Moreover, polyphenolic compounds not only play a role in controlling various related diseases to oxidative stress [9], but also regulate the activity of various cell receptors and enzymes [53].
Because the chemical composition of pomegranate peel is differ with the cultivar type such as sweet, sure, and sour-sweet [54,55], pomegranate antimicrobial activity may vary regarding its cultivar [56]. Tehranifar et al. (2010)
[57] reported that total polyphenols and tannins content in pomegranate juice were dependent on major chemical factors.
Kulkarni et al. [58] reported that antioxidant activity growth at the late-developmental phase was due to anthocyanins composition.
By investigating nine different cultivars, Shams Ardakani indicated that pulp of Sour Summer cultivar as a strong source of original antioxidants had the highest antioxidant activity than other cultivars (p < 0.05). The peel of Sour Summer, Sweet saveh malas, and Black peel is a considerable source of phenolic and flavonoid compound appropriate for phenolic and flavonoid purification and extraction. In addition, they reported that peel extracts had higher potential antioxidant activity and polyphenolic and flavonoid content than the pulps [38]. The pomegranate peel extract antioxidant capacity is 10 times greater than that of the pomegranate pulp extract. The North white peel and Black peel cultivar contain the highest flavonoid. Souleman et al. (2016) reported that seed of Egyptian pomegranate cultivar (PG1) contained the most total phenolic and flavonoids compounds (Table 3) [59].
Bassiri-Jahromi et al. (2015) [15] indicated that the peel extract of Saveh malas cultivar had the most effective element compared to other cultivars against Candida spp by MIC test [15]. Table 1 indicate that peel of Saveh malas pomegranate cultivar possesses a significant source of polyphenolic and flavonoid content compared to other cultivars. Difference in the pomegranate chemical compound is related to the cultivar, growing region, maturity, cultivation, climate, and storage situation [48]. This difference is also correlated with the latitude, altitude and longitude of growing regions [60]. The anthocyanin level of pomegranate juice variation was attributed to diversities of cultivars and growing region and various maturity levels of the pomegranate [40]. The pomegranate juice color is a significant index for juice quality; it is originally related to anthocyanin concentration.
Middha et al. [61] reported that higher total flavonoid concentration of pomegranate juice was almost correlated with sweet and sour cultivar and growing area. Although pomegranate anthocyanin pigments concentration decreased during 100 days, a considerable decrease in acidity was found as the significant chemical factor for increased incidence in over-ripe fruits.
This review clearly indicated that pomegranate peel extract possessed more natural antioxidants and activity as a health supplement than the pomegranate pulp extract. Derakhshani et al. in their study showed that the pomegranate peel extract contained high levels of antioxidant activity compared to seeds and juice in three different cultivars of various regions of Iran [62].
Table 1 shows the comparative evaluation of phenolic and flavonoids compounds attributes to various peels of pomegranate cultivars grown in the world.
These diversities may be owing to variety of cultivar, climate, edaphic condition, different maturity level, and particularly tannin specification method. Total tannin concentration pomegranate spectroscopic analyses by Khanavi et al. (2013)
[1] in Iran revealed that Black peel cultivar had the most hyperoside content in its pulp and peel. Hyperoside is identified as the significant flavonoid with respect to antioxidant activity. The results indicated that commercial pomegranate juice had significant phenolic quantity and antioxidant capacity. Furthermore, Zarei et al. (2010) revealed a significant difference in total phenolics, tannins, and antioxidant activity in the juice of six different Persian cultivars (Table 2) [63]. The polyphenolic content and antioxidant potent of the whole pomegranate juice were remarkably superior to aril juice of pomegranate from the same cultivar, due to the entrance of phenolic compounds from the rind sections of pomegranate to the juice [64].
Hajimahmoodi et al. [65] reported that pulp of Sour summer cultivar had the most antioxidant potent among the nine various pomegranate cultivars. The antioxidant capacity of pomegranate peel extract was 10 times higher than the pulp extract.
The antioxidant activity of pomegranate peels showed a rapid decrease in 20 to 60 days of fruit development (13%) [58]. Furthermore, Kulkarni et al. [58] reported a slight but important decrease in anthocyanin pigment after 100 days (9.3%). Moreover, they concluded that the anthocyanin increasing and phenolics decreasing were correlated with each other. In this regard, phenolics were exhausted when the anthocyanin pigment formation and the phenols were destroyed and their contents reduced [56].
Mphahlele et al. in their investigation showed that freeze-drying could be a viable method to proceed pomegranate peel to maintain the maximum natural value of their bioactive compounds [66].
4.
Conclusion
Different pomegranate cultivars had different polyphenol compositions and antioxidant potential. It is considerably associated with many factors such as cultivar type, growing region, maturity, cultivation, climate, edaphic condition, and storage situation [65]. They were also correlated with the latitude, altitude and longitude of growing regions. Additionally, difference in the average temperature and daily temperature during maturity and harvest period had significant effects on the total polyphenols, flavonoids and anthocyanin concentration of pomegranate.
Further future investigations are necessary to establish a database for pomegranate showing polyphenol and flavonoids compositions, antioxidant activities, physiochemical characteristics and their relation to environmental factors in various growing areas of the world. The data would be useful to produce better crops with higher nutritional quality. This database provides geographic product labeling and pomegranate brand identification.
Search strategy
This review is based on a method of systematic narrative review on comparative evaluation of bioactive compounds of various pomegranate cultivars. We conducted an extensive search using the PubMed, Web of Science, Science Direct, and Scopus databases in April 2018 to obtain related studies. This review aimed to present an overview of the comparison of the chemical analysis of total phenolic and flavonoids content of various pomegranate cultivars grown in different geographic regions of the world and to shows perspective advantages of pomegranate compound. It also aimed to summarize the present data from in vitro and in vivo tests animal trial systems and human clinical trials concerning the benefit of pomegranate compound. In addition, this review considered the pomegranate peel and pulp extracts activities and their future potential.
The findings of this review support that the pomegranate might be used in preventing and curing some diseases.
Research highlights
This study demonstrated considerable differences among the cultivars in most measured factors such as total poly phenolics, flavonoids, antioxidant activity and anthocyanins content. It also highlights the current aspects and the new research into the potential therapeutic pomegranate for some diseases and the future of clinical research of pomegranate.
Acknowledgments
We gratefully acknowledge the financial support of the Pasteur Institute of Iran Research Council (project No.TP-9003).
Conflict of interest
No author of this paper has a conflict of interest, including specific financial interests, relationships, and/or affiliations relevant to the subject matter or materials included in this manuscript.









 DownLoad:
DownLoad: