1. Introduction
A major challenge for this century is the transition from the economy based on fossil fuels to the sustainable and environmentally friendly economy based on renewable and clean resources which must be developed to meet the increasing energy demands of the world [1,2]. Hydrogen (H2) has been suggested as a principal, potential future energy carrier which would eventually lead to the implementation of the world-wide Hydrogen Economy [1,2]. That decisive shift would be allowed by applying hydrogen (H2) in a great variety of industrial and commercial applications.
In the transitional stage of transformation from the fossil fuels energy to hydrogen energy supply, a wide usage of fuel cells (FC) where hydrogen gas (H2) in contact with oxygen (O2) is converted into an electrical energy, must become a widespread reality. Furthermore, finding effective hydrogen storage systems for supplying FCs is another most important issue. There are three possible storage/generation systems for supplying H2 to a FC stack: gaseous H2, liquid H2 or solid hydrides. A mass transportation using cars (automotive) is the most important area where a fast transformation from fossil fuels to hydrogen would be most beneficial. For the automotive sector, solid state hydrogen storage in hydrides could be advantageous over gas and liquid storage because most solid hydrides exhibit a higher H2 volumetric density than gas or liquid storage [3,4,5,6]. In addition, such serious safety problems like a very high pressure of 70 MPa for H2 gas storage or large thermal losses for liquid H2, which requires a formidable insulation and an open storage system [3,4,5,6], could be eliminated. In addition, substantial pressurization or liquefaction costs could be avoided.
However, solid state H2 storage in hydrides has its own serious constraints. First, reaction of H2 and oxygen (O2) in a commercial high power density Proton Exchange Membrane fuel cell (PEM FC) (sometimes also named a Polymer Electrolyte Fuel Cell (PEFC)) imposes important constraints on solid hydrides for potential H2 storage. A large PEM FC stack (a few hundred cells) generates the quantity of waste heat which is able to rise coolant temperature to 70-80 °C. Second, a PEM FC operates at H2 fuel pressure slightly above 1 bar (usually 1.1-1.8 bar). Therefore, the suitable solid hydrides for generating H2 gas should rapidly desorb H2 under pressure levels roughly 1.0-1.8 bar and at low temperatures, not exceeding the waste heat temperature of a FC stack (60-80 °C) which, in turn, could be used for heating a hydride storage tank to release H2. Therefore, for the preliminary, experimental screening of hydrides, the hydride systems that are of potential interest for solid state hydrogen storage, including automotive applications, are only those that would desorb H2 under at least 1 bar H2 pressure (or slightly higher) and at temperatures not exceeding approximately 100 °C.
The second constraint is a requirement for a reasonable driving distance. The US Department of Energy target for 2017 is 5.5 wt.% H2 for the entire fuel system [7] to drive 300 miles (480 km), which roughly corresponds to at least 10-11 wt.% H2 necessary capacity for the desorbing material/hydride. Furthermore, the potential automotive solid state hydride storage systems would have to be easily reversible by allowing “on board” rehydrogenation under medium H2 pressures, at a charging time not exceeding approximately 3.3 min [7] and within a temperature range not exceeding the waste heat temperature of a FC stack (70-80 °C). Unfortunately, despite enormous research efforts in the past 15 years a high capacity hydride storage system specifically suited for an automotive market is still very elusive and there is a growing doubt if it would ever be found [5,6,8]. Since the automoti ve solid state H2 storage is still very futuristic, the first commercial Toyota and Hyundai Fuel Cell Vehicles (FCV), coming to market in 2015-2016 [9,10] will store H2 in a more conventional, gaseous form under a high pressure of 70 MPa.
However, there is a number of other potential market applications for H2 generation systems at ambient and slightly elevated temperatures. Those H2 systems became available in the past years by novel processing and synthesis methods, that could enhance a gradual implementation of the Hydrogen Economy in the commercial, non-automotive sectors of the economy. They could be utilized in such applications as, for example, portable electronic devices, stationary auxiliary power systems, off-road vehicles, lawn mowers, air transportation, coastal and international shipping, bulk hydrogen storage and many others which will be discussed in more detail later on.
The present review focuses on the results obtained hitherto in our laboratory in the past few years on various types of solid hydride H2 generators of principally non-automotive character. These hydride systems have been synthesized by controlled ball milling under varying energy levels using the magneto-mill Uni-ball Mill 5 [11,12,13] resulting in some novel hydrides formed by mechano-chemical activation synthesis (MCAS) involving metathesis or, in other words, double substitution reactions. The microstructure and properties for hydrogen generation of these novel systems will be discussed. The experimental details of the processing have already been published in a number of publications and will not be repeated in the present work. Readers are encouraged to refer to the original publications. It must be pointed out that ball milling leads to the formation of nanostructure in the hydride systems. Either powder nanoparticles or crystallites (nanograins) within the larger powder particles are being formed during ball milling [3]. These aspects of microstructure will also be discussed for each hydride system described here wherever possible. Since in the presented hydride systems either additives or hydrides are nanometric we will use a term “nanocomposite”. Finally, for clarity, the quantities of wt.% H2 released which are reported in the present work are always referred to the whole mass of the solid powder, i.e. hydride with all additives.
2. Hydrogen Generators Through Mechanical Dehydrogenation at the Ambient Temperature
2.1.LiAlH4 with various additives
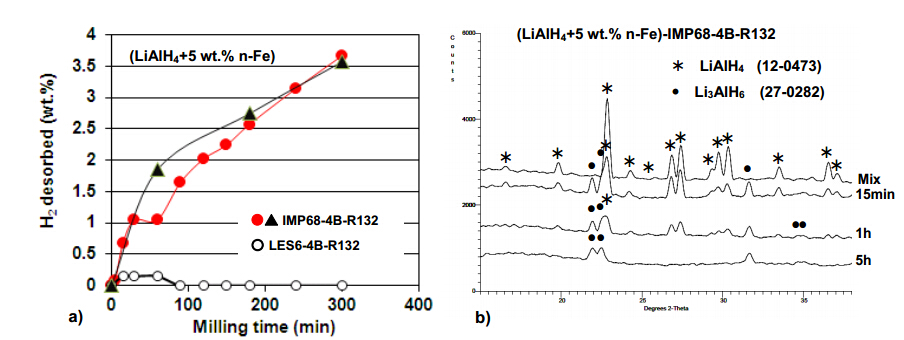
Recently, we have found that the lithium alanate (LiAlH4) hydride, ball milled together with the nano-iron (n-Fe) particles additive (average particle size d50 < 50 nm; specific surface area (SSA) ~25 m2/g) under a high energy milling mode starts decomposing rapidly during milling, releasing large quantities of H2 (Figure 1a) up to about 3.5 wt.% after 5 h (300 min) of ball milling [4,14]. This is a typical phenomenon of “mechanical dehydrogenation” that will be also shown and discussed for LiAlH4 mixed with other additives and complex borohydride systems. An enhanced mechanical dehydrogenation has never been reported for the n-Fe additive to LiAlH4. It must be stressed out that no H2 desorption is observed during low energy milling of LiAlH4 containing n-Fe under a low energy shearing mode (Figure 1a). Similarly, no H2 desorption occurred during high energy ball milling for LiAlH4 containing micrometric Fe (μ-Fe) and, for comparison, both the micrometric and nanometric Ni (μ-Ni and n-Ni) additive [4,14]. Figure 1b c ompares the XRD patterns obtained from the powders milled under a high energy mode for 15 min, 1 h and 5 h with the XRD pattern for just mixed powder. After only 15 min of milling the diffraction peaks of Li3AlH6 are clearly seen as opposed to the LiAlH4 peaks observed for the mixed powders. The intensity of the Li3AlH6 peaks increases after 1 and 5 h of milling while the intensity of LiAlH4 peaks gradually decreases and they eventually disappear (Figure 1b). Simultaneously, more H2 is desorbed as can be seen in Figure 1a. It is obvious that during high energy milling there is a gradual decomposition of LiAlH4 in solid state according to reaction described by Equation (1):
LiAlH4(s)®1/3Li3AlH6(s)+2/3Al(s)+H2(g) (1)
where s-solid and g-gas. The theoretical H2 capacity of this reaction is 5.3 wt.% H2.
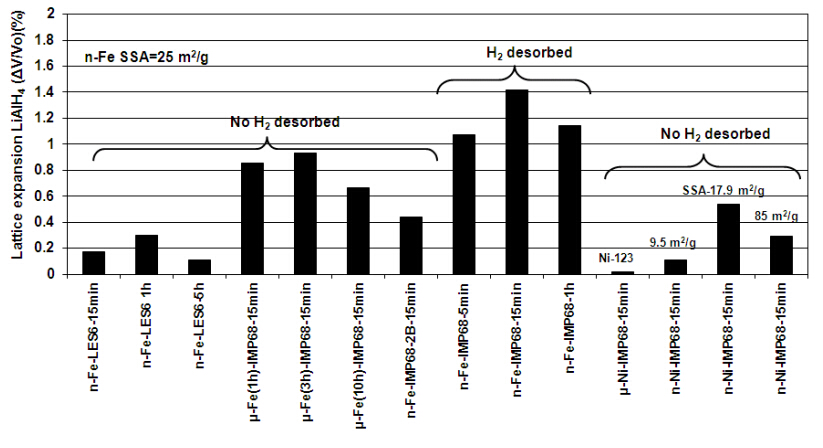
We have also found from X-ray diffraction that ball milling results in a varying degree of the lattice expansion of LiAlH4 for both the nanometric Fe and Ni (n-Fe and n-Ni) additives as shown in Figure 2. It seems that a volumetric lattice expansion larger than 1% triggers the accelerated decomposition of LiAlH4 accompanied by a continuous H2 desorption during milling according to Equation (1). It is hypothesized that the Fe and Ni ions are able to dissolve in the lattice of LiAlH4 by the combined action of mechanical energy and chemical reaction, forming a substitutional solid solution of either the LiAlFexH4 or LiAlNixH4 type [4,14]. The ionic radius of 77 pm for Fe2+ is much larger than 53 pm for Al3+ [15] and the LiAlH4 lattice expansion increases. In contrast, the ionic radius of Ni2+ is 69 pm [15] and the volumetric lattice expansion is smaller (Figure 2). It is interesting that the profound dissolution of the Fe2+ ions in the LiAlH4 matrix can only occur when the Fe additive is nanometric while it does not occur if the additive is micrometric in size [4,14]. Furthermore, the experimental results in Figure 2 show that n-Fe is more efficient in enhancing the dehydrogenation of LiAlH4 during ball milling than n-Ni. This is a puzzling finding because the Ni2+ ionic radius is smaller than that of the Fe2+ ion and thus, one would expect more Ni2+ ions dissolved in the LiAlH4 lattice. Apparently, for the same milling conditions, the incorporation of Ni2+ ions into the ionic-covalent crystal of LiAlH4 is less efficient than the Fe2+ ions as can be seen in Figure 2. Therefore, it was suggested that an efficient incorporation of metal atoms/ions in the LiAlH4 lattice could be driven by chemical reactions with use of oxide form of the alloying metal, instead of the metal itself [4,14]. Indeed, the received n-Fe (NANOFER 25S from the Nano Ir on, s.r.o., The Czech Republic) contained Fe3O4 [4,14]. Finally, it must be pointed out that the mechanism of accelerated mechanical dehydrogenation of LiAlH4 (Figure 1) seems not to be a typical catalytic mechanism in which a catalyst is supposed to boost the surface formation of molecular H2 from the atomic hydrogen escaping from the bulk [3,4].
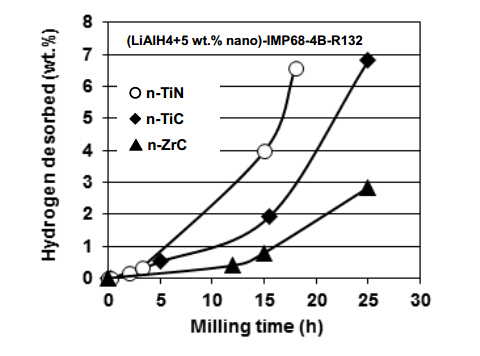
Another example of a rapid mechanical dehydrogenation of LiAlH4 is the addition of 5 wt.% nanometric interstitial compounds such asn-TiC (average particle size 40 nm; specific surface area (SSA) = 50 m2/g), n-TiN (average particle size 20 nm; SSA >80 m2/g) and n-ZrC (average particle size 40 nm; SSA > 70 m2/g) [16]. The interstitial compounds are composed of the transition metals (T) with relatively large atomic radii with nonmetals having small radii (H, B, C, N and O) [17]. Figure 3 shows the quantity of H2 desorbed during ball milling of LiAlH4 containing 5 wt.% of the interstitial compound additive. After about 18 h of milling the amount of mechanical dehydrogenation for the n-TiN additive reaches ~6.6 wt.% H2. Finally, after 25 h of milling mechanical dehydrogenation results in ~7 wt.% H2 desorbed for the n-TiC additive, and ~3 wt.% H2 for the n-ZrC additive. Therefore, the mechanical dehydrogenation curves in Figure 3 confirm that the mechanical dehydrogenation rate of the samples containing the interstitial compound additives increases noticeably during high energy ball milling with increasing quantity of injected energy in the order of n-TiN > n-TiC > n-ZrC. Since the maximum theoretical H2 capacity of reaction Equation (1) is only 5.3 wt.% H2 then desorption of ~7 wt.% H2 during the mechanical dehydrogenation o f LiAlH4 with n-TiN and n-TiC requires another reaction to occur according to Equation (2) [3,4,16]:
1/3Li3AlH6 ® LiH + 1/3Al + 0.5H2 (theoretical 2.6 wt.% H2) (2)
The combined theoretical capacity for reactions described by Equation (1) and (2) is 7.9 wt. H2 and the practical H2 capacity is ~7.3 wt.% if both the purity and presence of 5 wt.% additive are taken into account (4.9 and 2.4 wt.% H2 for reactions (1) and (2), respectively [16]). Figure 3 shows that nearly 55% of the maximum available H2 capacity of the (LiAlH4 + 5wt.% n-TiN) nanocomposite can be desorbed during milling up to 15 h, while the nanocomposites with 5 wt.% n-TiC and n-ZrC release almost 23% and 10% of their respective maximum available capacity after the same milling time, respectively. The upward trend of desorption curves in Figure 3 suggests that applying longer milling duration could, most likely, result in a total H2 desorption from the catalyzed nanocomposites. The XRD measurements confirmed the correctness of reactions Equation (1) and (2) [16]. The examination of the XRD patterns after various ball milling durations also confirmed a continuous presence of well visible diffraction peaks of nanometric interstitial compounds. This observation provides strong evidence that they do not decompose during high energy ball milling and thus exhibit a considerable chemical stability. Furthermore, calculations of the unit cell volume of LiAlH4 after ball milling showed no measurable change in the unit cell volume [16]. It is quite apparent that accelerated mechanical dehydrogenation for LiAlH4 containing nanometric interstitial compounds in Figure 3 is unrelated to the lattice expansion that might have been induced by, for example, diffusion of the Ti or Zr ions from the nanometric interstitial compounds into the LiAlH4 crystal lattice as shown earlier for the n-Fe additive. The absence of a measurable lattice expansion is in agreement with the experimental fact that the nanometric interstitial compounds are very stable during high energy ball milling and do not decompose releasing the Ti or Zr ions. Furthermore, since the average number of valence electrons per atom, or valence electron concentration (VEC), for TiC and ZrC is VEC = 8 and TiN has VEC= 9 [17], respectively, but the n-ZrC additive exhibits the weakest catalyzing effect (Figure 3) then their catalytic activity for mechanical dehydrogenation seems not to be related to their VEC number. It must, however, be pointed out that the n-TiN additive has the smallest average particle size of 20 nm and the largest SSA > 80 m2/g. It also exhibits the fastest mechanical dehydrogenation rate in Figure 3. In turn, both n-TiC and n-ZrC have the average particle size on the order of 40 nm and n-TiC and n-ZrC has SSA = 50 m2/g and >70 m2/g, respectively. However, despite that the SSA for n-ZrC is larger than that for n-TiC the mechanical dehydrogenation rate is higher for n-TiC than that for n-ZrC (Figure 3). Therefore, it seems that SSA or a particle size is not a major factor for the catalytic efficiency of n-TiC and n-ZrC. It was proposed [16] that the primary factor responsible for a strong catalytic effect of the nanometric n-TiN interstitial compound during mechanical dehydrogenation is its very small particle size on the order of 20 nm and the largest SSA > 80 m2/g. The secondary factor, at a nearly equal particle size or SSA, seems to be a stron ger catalytic effect for Ti than that for Zr in an interstitial compound.
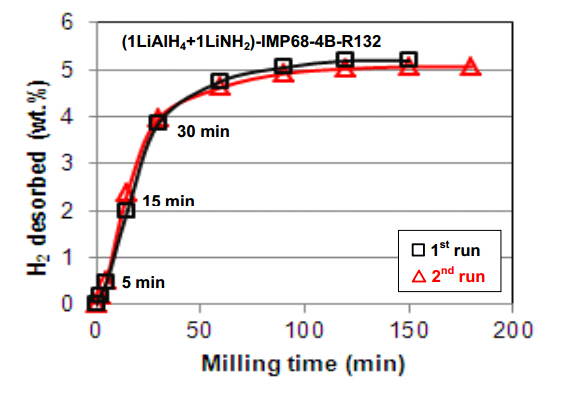
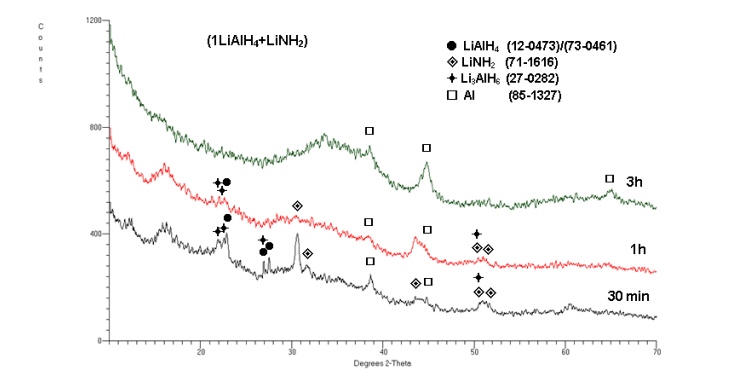
In the cases discussed above a very efficient mechanical dehydrogenation phenomenon was induced by small amounts (5 wt.%) of non-hydride additives which were ball milled with the LiAlH4 matrix. However,we also observed quite rapid mechanical dehydrogenation of the system in which LiAlH4 was ball milled in a molar ratio 1:1 with a lithium amide (LiNH2) hydride (1LiAlH4 + 1LiNH2) [18]. Figure 4 shows that after 30 min (0.5 h) and 150-180 min (2.5 and 3h) of ball milling the system releases as much as 4 and 5 wt.% H2,respectively. We repeated the milling test in Figure 4 twice (1st and 2nd run) to confirm that the results are reproducible. As can be seen,the hydrogen desorption during ball milling is perfectly reproducible and the quantity of desorbed H2 is nearly exactly the same for the 1st and 2nd run. For clarity,it is to be pointed out that the quantity of released H2 rapidly decreased for the molar ratio (3LiAlH4 + LiNH2) and was not observed at all for the ratios 11.5LiAlH4 + LiNH2) and (30LiAlH4 + LiNH2) [18]. The (1LiAlH4 + 1LiNH2) hydride-hydride system has much higher initial rate of H2 release (Figure 4) than the previously discussed systems of (LiAlH4 + 5wt.% n-Fe)(Figure 1a) and (LiAlH4 + 5 wt.% n-TiN/n-TiC/n-ZrC)(Figure 3).
In other words,the propensity of the lithium alanate-lithium amide system for mechanical dehydrogenation substantially decreases with increasing molar ratio of LiAlH4 in the mixture. Since for the molar ratio (1LiAlH4 + 1LiNH2) the mass content of LiNH2 in the mixture equals 37.7 wt.% it means that this particular mass content of LiNH2 in the mixture is a minimum required for efficient mechanical dehydrogenation. This behavior is quite puzzling. In order to obtain more insight into the phase changes occurring during ball milling as a function of milling time,X-ray diffraction (XRD) measurements were carried out on the composite samples extracted after pre-determined milling durations which are presented in Figure 5. As can be seen with increasing ball milling time to 0.5 (30 min),1 and 3 h,the peaks become broadened and diffused as well as a pronounced uprising in the baselines and the formation of a broad “hump” in the range of 2θ = 30-40° are observed which can be attributed to heavy nanostructuring or even the existence of increasing quantities of amorphous structure(s) [18]. The 100% intensity peaks for LiAlH4,LiNH2 and Li3AlH6 are visible after 0.5 and even after 1 h of milling although much weakened. There are also clear peaks which we assigned to Al. The presence of Li3AlH6 and Al in the microstructure of mechanically dehydrogenated ball milled samples clearly indicate that at least reaction described by Equation (1) must have occurred during ball milling. Furthermore,since the molar ratio (1LiAlH4 + 1LiNH2) corresponds to a weight ratio of 62.3 wt.% LiAlH4 and 37.7 wt.% LiNH2,thus,at this weight ratio,fully completed reactions described by Equation (1) and (2) should provide approximately purity-corrected 3.2 and 1.6 wt.% H2,respectively [18]. Since ~5 wt.% H2 is released after 150 min ball milling (Figure 4) that means that both reactions must have occurred during ball milling. As pointed out in [18] this is a peculiar observation because in a thermal DSC test,reaction described by Equation (1) is exothermic and that by Equation (2) is endothermic [3,4,19,20] which would suggest that both types of reactions,thermodynamically opposite,have been realized during high energy ball milling. However,since during ball milling the overall temperature increase in the milling vial is very small then the question arises whether the thermodynamic character of both reactions,which are now induced by mechanical energy,remains the same as that at elevated temperatures during DSC runs. Furthermore,it seems that the entire quantity of H2 desorbed during ba ll milling of the 1:1 mixture (Figure 4) can be easily provided by reactions described by Equation (1) and (2). It was also argued in [18] that since the diffraction peaks of LiNH2 in the 1:1 mixture persist throughout a long period of milling (Figure 5),it strongly indicates that LiNH2 does not react/decompose but most likely becomes heavily nanostructured or even amorphized and in this capacity destabilizes LiAlH4 during ball milling and enhances its decomposition according to reaction described by Equation (1) and (2) without involvement of any other reactions. However,as mentioned earlier,with decreasing molar content of LiNH2 in the mixture,LiNH2 somehow ceases to destabilize LiAlH4 during ball milling. At the moment it is difficult to propose any detailed molecular mechanism by means of which the peculiar content of LiNH2,equal to 37.7 wt.% for a 1:1 ratio,profoundly destabilizes LiAlH4 during ball milling and,furthermore,the dependence of that mechanism on the molar ratio of the composite [18].
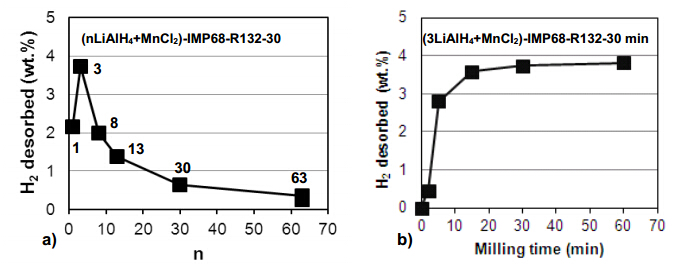
Recently,we have investigated the effect of a manganese chloride (MnCl2) additive on the hydrogen storage properties of LiAlH4 for a wide range of molar ratios n in the (nLiAlH4 + MnCl2) system where n= 1,3,6,8,13,30 and 63 (5 wt.% MnCl2) [4,21]. This chloride is less volatile than those of Ti,V and Zr metals. Figure 6a shows that for the low molar ratios n = 1 to 13,the system releases substantial quantities of H2 during ball milling with the maximum for n = 3. When the molar ratio n increases to 30 and 63 the quantity of released H2 dramatically decreases. Figure 6b shows that for n = 3 ball milling up to barely 0.5 h (30 min) results in nearly 4 wt.% H2 desorbed. At the first look this behavior is very similar to that for (1LiAlH4 + 1LiNH2) shown in Figure 4,in the sense,that the rate of mechanical dehydrogenation is rapid in the beginning of ball milling and subsequently saturates.
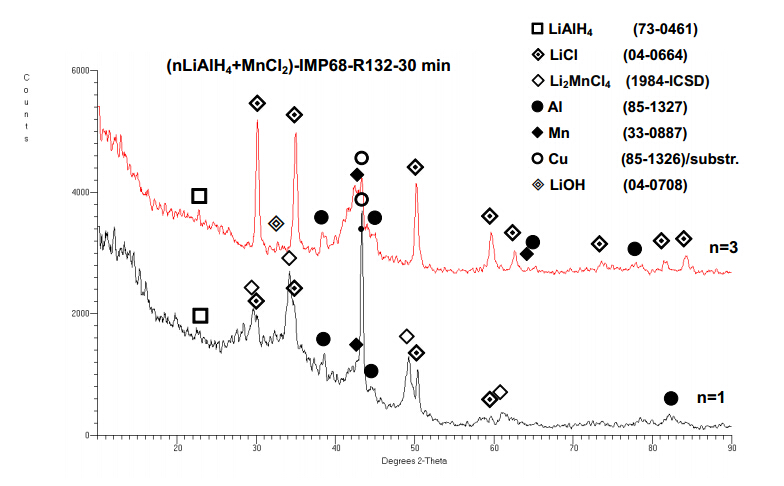
Figure 7 shows the XRD patterns of the mixtures with the molar ratios n = 1 and 3 after ball milling for 0.5 h. The peak of copper (Cu) in both patterns arises from a support plate of the XRD sample holder used [4,21]. In the n = 1 mixture the principal diffraction peaks of the Li2MnCl4 compound are accompanied by minor peaks of LiCl,Al and a very broadened Mn peak which suggests that the Mn metal is heavily nanostructured. The Li2MnCl4 compound is an inverse cubic spinel that has been extensively studied for its ionic conductivity as a potential solid electrolyte for lithium-ion batteries [4,21]. Its formation during ball milling in the (nLiAlH4 + MnCl2) system has never been reported in the literature. The presence of Li2MnCl4,Al and Mn,combined with the fact that about 2 wt.% H2 was desorbed within 0.5 h of ball milling (Figure 6a; n = 1),strongly suggests that following reaction must have occurred during mill ing for the molar ratio n = 1:
LiAlH4 [4,21]. Apparently,the presence of these phases suggests the following reaction:
The total theoretical H2 capacity of reaction described by Equation (3) is 2.46 wt.%. However,the presence of the LiCl peaks which form doublets with the Li2MnCl4 peaks in Figure 7 also suggests that another reaction was taking place during ball milling:
2LiAlH4 + MnCl2®2LiCl + 2Al + Mn + 4H2 (4)
Reaction described by Equation (4) has a theoretical H2 capacity of 4 wt.%. It is not clear why this reaction occurred for the molar ratio n = 1. Normally,it should have occurred for the molar ratio n = 2. Perhaps,small powder volumes during milling were locally enriched to the n = 2 ratio. Figure 6a shows that about 2 wt.% H2 was desorbed during milling for n= 1. That means that the principal reaction releasing H2 was likely reaction described by Equation (3) with only a small fraction of H2 released from reaction described by Equation (4). As shown in Figure 7 ball milling of the n = 3 system for 0.5 h produced principal phases LiCl,Mn,Al and a minority phase LiAlH4. The observed weak peak of minor phase LiOH for n = 3 is an impurity commonly found in commercial
LiAlH4 [22,23]. Apparently,the presence of these phases suggests the following reaction:
3LiAlH4 + MnCl2®LiAlH4 + 2LiCl + 2Al + Mn + 4H2 (5)
The theoretical capacity of this reaction is 3.4 wt.% H2 and slightly less taking into account the purity of reactants (97% for LiAlH4 and 99.99% for MnCl2). The quantity of H2 desorbed within 0.5 h of milling for n = 3 in Figure 6b is 3.7 wt.% which means that all H2 from reaction described by Equation (5) was already desorbed during milling. The XRD patterns for the n = 6,8,13,30 and 63 (5 wt.% MnCl2) molar ratio always show the same phases LiCl,Mn,Al and LiAlH4 after ball milling for 30 min [4,21],associated with H2 release (Figure 13a). That means that for n ³ 2 reaction described by Equation (5) can be written in the following general form:
nLiAlH4 + MnCl2®(n-2)LiAlH4 + 2LiCl + 2Al + Mn + 4H2 (6)
Apparently,in this case the H2 desorption during ball milling occurs owing to simple chemical reaction in which LiAlH4 is gradually consumed by reacting with MnCl2 to produce LiCl,Al,Mn,possibly in a nanostructured form,and H2. However,for n = 1 in addition to Al,Mn and LiCl an inverse cubic spinel phase,Li2MnCl4,is formed as a result of mechanical dehydrogenation according to Equation (3).
2.2.LiNH2-MgH2
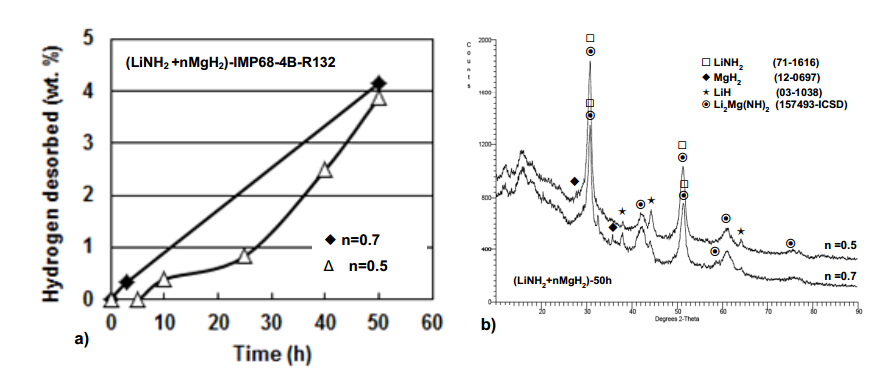
The (LiNH2 + nMgH2) system is a hydride/hydride system that also exhibits the phenomenon of mechanical dehydrogenation during ball milling. Figure 8a shows the quantity of H2 desorbed during ball milling for the (LiNH2 + nMgH2) system where n = 0.5 and 0.7 as a function of milling time. After 50 h of ball milling nearly 4 wt.% H2 is desorbed as a result of mechanical dehydrogenation. In contrast to the previously discussed H2 generating systems the rate of mechanical dehydrogenation for the (LiNH2 + nMgH2; n = 0.5 and 0.7) system is the lowest one of all investigated systems based on LiAlH4. In the order of decreasing mechanical dehydrogenation rate,the systems can be ranked as follows: (1LiAlH4 + 1LiNH2) in Figure 4 (4 wt.% H2 within 0.5 h),(3LiAlH4 + MnCl2) in Figure 6a (4 wt.% H2 within 1 h),(LiAlH4 + 5 wt.% n-Fe) in Figure 1a (3.6 wt.% H2 within 5 h),(LiAlH4 + 5 wt.% n-TiN/n-TiC) in Figure 3 (4 wt.% H2 in 15 h and 6.6 wt.% H2 within 18 h) and (LiNH2 + nMgH2; n = 0.5 and 0.7) in Figure 8a (4 wt.% H2 within 50 h). Figure 8b shows the XRD patterns for the (LiNH2 + nMgH2) system (n = 0.5 and 0.7) ball milled for 50 h after release of about 4 wt.% H2 as shown in Figure 2c. Both,the XRD in Figure 8b and FT-IR patterns (not shown here) indicate clearly that the following reactions occurred during milling [4,21,22]:
LiNH2 + nMgH2®0.5Mg(NH2)2 + 1.0LiH + (n−0.5)MgH2 (7a)
0.5Mg(NH2)2 + 1.0LiH + [(n-0.5)MgH2]®0.5Li2Mg(NH)2 + [(n−0.5)MgH2] + 1.0H2 (7b)
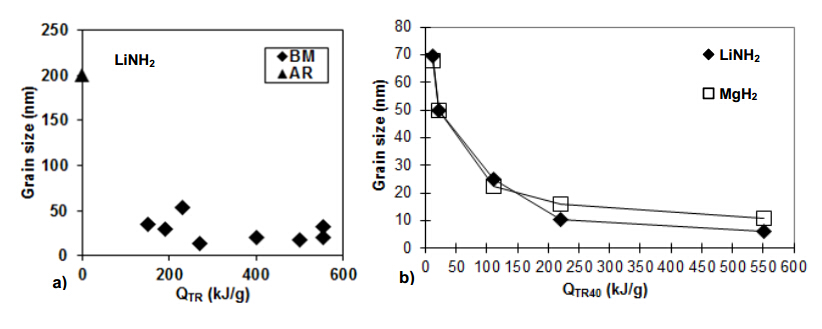
The milling process depends on a number of parameters such as the geometry of milling device,milling mode,ball-to-powder weight ratio (R),dimensions of milling balls and milling time. This makes a direct comparison of milling results rather difficult. For our magneto-ball mill,the Uni-Ball-Mill 5,in which the milling processing discussed in this work was carried out,we derived by a semi-empirical method the universal milling parameter which is the milling energy input in kJ per unit mass (g) of powder,QTR (kJ/g),as described in detail in [22,23]. Therefore,QTR is a single,universal parameter by means of which any milling mode can be compared with another one after a specified milling time for a particular hydride composite system. Figure 9a shows the overall grain size changes for various milling modes and R ratios as a function of injected energy input (QTR). It is clearly seen that the LiNH2 grain size decreases rapidly with increasing QTR reaching the size range 10-50 nm at the QTR = 150-200 kJ/g and subsequently more or less saturates. Figure 9b shows the variations of grain size for LiNH2 and MgH2 constituents for a fixed molar ratio n = 0.7 (LiNH2 + 0.7MgH2) and a constant ball-to-powder mass ratio,R = 40,as a function of injected energy QTR40. The crystallite (grain) size decreases rapidly with increasing QTR up to approximately 150-200 kJ/g and subsequently more or less saturates at the value of 10-20 nm. Also,Figure 9a shows that a relatively low QTR is sufficient to reach the grain size of 100 nm for LiNH2 which is considered a threshold value for attaining a nanocrystalline structure [22]. The lattice strain has been found to be minimal (0.12-0.49%) [22].
2.3. Complex borohydride synthesized by mechano-chemical activation synthesis (MCAS)
Some of those new complex borohydride systems are derivatives of lithium borohydride (tetrahydroborate),LiBH4,which is a commercially available,high capacity complex metal hydride having the highest theoretical gravimetric H2 capacity (18.5 wt.%) and good volumetric capacity (122.5 kgH2/m3) [24]. It is well established [24,25] that at around 100-118 °C,LiBH4 undergoes a polymorphic transformation from the orthorhombic Pnma crystal structure to the hexagonal P63mc crystal structure which is followed by melting around 280 °C and subsequent dehydrogenation reaction in the following form:
LiBH4 (l)®LiH (s)+ B (s) + 3/2H2 (g) (8)
where l-liquid,s-solid and g-gas. The theoretical maximum quantity of H2 desorbed in Equation (8) is 13.9 wt.%. Since LiH is very stable and can only be decomposed at temperatures in excess of
700 °C [25] the rest of the total H2 capacity from LiBH4 is not easily accessible.
To complicate the case even more,the enthalpy and entropy changes (ΔH and ΔS) for reaction (1) are reported as ΔH = 68.9 kJ/molH2 and ΔS = 100.2 J/KmolH2 [26] and ΔH = 74 kJ/molH2 and ΔS = 115 J/KmolH2 [27] which indicate that reaction described by Equation (8) is endothermic. According to the Van’t Hoff equation [3,4,5]:
ln (p/p0) = -ΔH/RT+ ΔS/R (9)
However,LiBH4 can be destabilized in solid state by transforming it into other complex metal hydrides,more suitable for H2 generation. In these cases a mechano chemical activation synthesis (MCAS) by ball milling was used to induce chemical reactions between LiBH4 and an appropriate metal chloride (MCln; M-metal) which resulted in the formation of new metal borohydrides,always mixed with LiCl,the latter being a by-product of MCAS reaction [28,29].
However,LiBH4 can be destabilized in solid state by transforming it into other complex metal hydrides,more suitable for H2 generation. In these cases a mechano chemical activation synthesis (MCAS) by ball milling was used to induce chemical reactions between LiBH4 and an appropriate metal chloride (MCln; M-metal) which resulted in the formation of new metal borohydrides,always mixed with LiCl,the latter being a by-product of MCAS reaction [31].
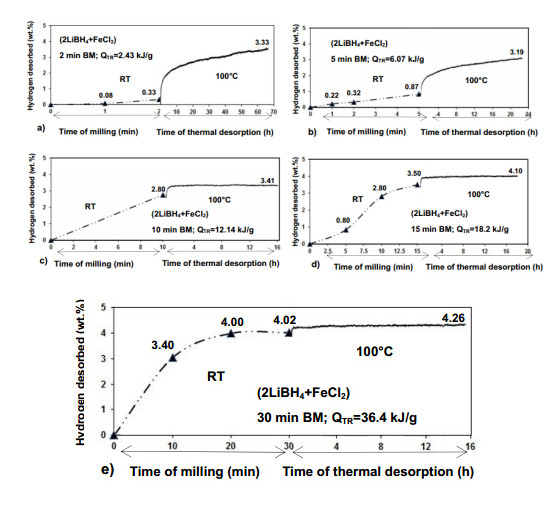
In due course of our experimental work on fast hydrogen generating hydrides (or their mixtures) mentioned above,we recently noticed a high proclivity of the (2LiBH4 + FeCl2) hydride/chloride system to rapidly generate H2 during ball milling at the ambient temperature,without any external heating [30]. Figure 10 shows the effects of increasing milling energy input,QTR,on the mechanical dehydrogenation of samples. A minimal amount of 0.33 wt.% H2 is released due to mechanical dehydrogenation after injection of 2.43 kJ/g milling energy (Figure 10a) which increases to 0.87 wt.% H2 after injecting nearly 3-fold quantity of milling energy (6.07 kJ/g)(Figure 10b). The quantity of H2 due to mechanical dehydrogenation rapidly increases to 2.80 (Figure 10c),3.50 (Figure 10d) and 4.02 wt.% (Figure 10e) after injecting 12.14,18.2 and 36.4 kJ/g of milling energy input,respectively. Since the total theoretical H2 capacity of the (2LiBH4 + FeCl2) mixture is 4.73 wt.%,injecting barely 36.4 kJ/g of milling energy input (0.5 h (30 min) ball milling under the mode shown) leads,at the ambient temperature,to mechanical dehydrogenation of about 85% of the theoretical H2 capacity (Figure 10e),or even slightly higher if one takes into account the purity corrected total capacity. It is also clear that if the H2 quantity released by mechanical dehydrogenation is approximately below 4 wt.% (Figure 10a-d),subsequent thermal dehydrogenation of the ball milled samples releases slightly more H2 although with a dehydrogenation rate lower than that for mechanical dehydrogenation. Apparently,the mechanical dehydrogenation rate at the ambient temperature is found to be much higher than the thermal dehydrogenation rate at the 100 and 250 °C range (not shown here). To achieve that remarkable H2 desorption rate the required milling energy input is very small,36.4 kJ/g which is another beneficial aspect of the investigated solid state chemical system. This is a very beneficial behavior,indeed,for a rapid H2 generator,clearly indicating that elevated temperature is not really needed for desorbing large quantities of H2 but just a very small quantity of mechanical energy is sufficient.
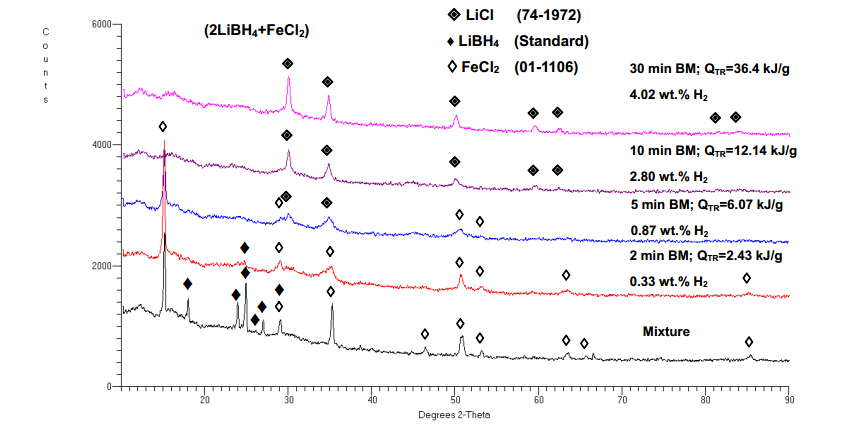
Figure 11 shows the XRD pattern as a function of milling energy input (milling time) for samples whose mechanical dehydrogenation curves are shown in Figure 10. It is clearly seen that the peaks of LiBH4 disappear just after the milling energy input reaches 2.43 kJ/g while the peaks of FeCl2 disappear after about 12.14 kJ/g of milling energy input. The peaks of LiCl become visible after the injection of 6.07 kJ/g milling energy. Such a rapid disappearance of the LiBH4 peaks without yet visible formation of LiCl suggests that LiBH4 becomes highly disordered by ball milling. The presence of LiCl after ball milling,which is not accompanied by any other diffraction peaks,strongly indicates that metathesis (double substitution) reaction occurred between LiBH4 and FeCl2 forming LiCl and unknown hydride that does not have a regular crystallographic structure that would allow X-ray diffraction. Furthermore,this hydride subsequently decomposes during continuous ball milling releasing hydrogen. This observation is in a good agreement with Nakamori et al. [28,29] who reported formation of disordered hydrides M(BH4)n (M-metal) that did not produce any diffraction patterns when they used metal chlorides,MCln where M = Cr,Mg,Mn,Zr,Ti,V,Zn,Ca,Sc,Al for mechano-chemical activation synthesis. According to Nakamori et al. [28,29] those disordered complex metal hydrides do not exhibit a long-range order of the Mn+ and (BH4)n− units but they do have a short-range order. Taking into consideration our XRD results in Figure 11 and a couple papers on either wet reaction of LiBH4 and FeCl2 in diethyl ether [31] or mechano-chemical synthesis [32],we hypothesize that a rapid rate of mechanical dehydrogenation is the result of the formation of a metastable,disordered iron borohydride,d-Fe(BH4)2,which rapidly decomposes during continuous ball milling (BM) releasing H2 according to following reaction:
LiBH4 + 0.5FeCl2®(BM)®0.5d-Fe(BH4)2 + LiCl®(BM)®0.5a-Fe + a-B + 2H2 + [LiCl] (10)
It is to be pointed out that all ball milled samples exhibit ferromagnetic behavior being strongly attracted to a magnet. This is good supporting evidence of the presence of α-Fe in the microstructure. Also,Fourier transform-infrared (FT-IR) spectra show the presence of a new borohydride,most likely,d-Fe(BH4)2 after ball milling [30].
So far,the (2LiBH4 + FeCl2) system exhibits the highest rate of mechanical dehydrogenation releasing 4.02 wt.% H2 after injecting a very small milling energy input of barely 36.4 kJ/g (0.5 h milling). For comparison,the earlier discussed (1LiAlH4 + LiNH2) hydride/hydride (Figure 4) and (3LiAlH4 + MnCl2) hydride/halide (Figure 6) systems were capable of mechanical dehydrogenation of 3.7 wt.% H2 after injecting the same 36.4 kJ/g quantity of milling energy input. The other hydride systems,already discussed,exhibit a much lower mechanical dehydrogenation rate. The quantity of 4 wt.% H2 released from the (LiAlH4 + 5 wt.% n-Fe),(LiAlH4 + 5 wt.% n-TiN) and (LiNH2 + (0.5 and 0.7) MgH2) hydride systems,required a milling energy input of 364 (Figure 1a),1090 (Figure 3) and 3600 kJ/g (Figure 8a),respectively,converting the milling time required for the release of 4 wt.% H2 into the energy input as described in Sec. 2.2.
3. Hydrogen Generating Systems at Low Elevated Temperatures (100 °C)
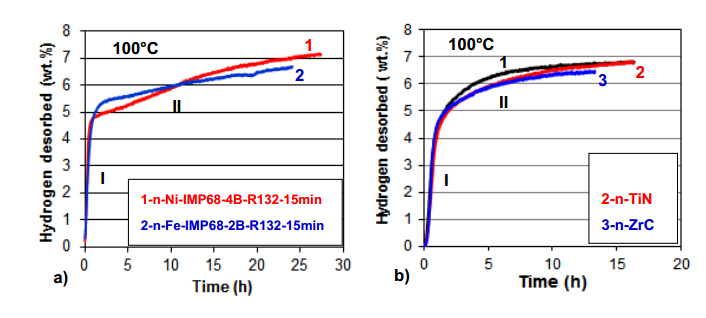
A LiAlH4 hydride with catalytic metal and interstitial compounds additives,which was discussed in the preceding sections,in its capacity as a rapid H2 generator through mechanical dehydrogenation during ball milling,is also capable of desorbing H2 at temperatures that do not exceed 100 °C. In this case the LiAlH4 mixture with additives must be ball milled under conditions under which no mechanical dehydrogenation occurs. Figure 12 shows volumetric dehydrogenation curves at 100 °C for LiAlH4 with added 5 wt.% n-Ni [19] and n-Fe [14] (Figure 11a),and 5 wt.% n-TiC/n-TiN/n-ZrC (Figure 11b) [16] which were ball milled with the milling energy input QTR = 18.2 kJ/g (IMP68-4B-R132-15 min) [19],QTR = 18.4 kJ/g (IMP68-2B-R132-15 min)[14] and QTR = 18.2 kJ/g (IMP68-4B-R132-15 min) [16],respectively. This small quantity of injected milling energy input did not induce mechanical dehydrogenation. As can be seen,the ball milled nanocomposite (LiAlH4 + 5 wt.% n-Ni/n-Fe) is capable of desorbing close to 7 wt.% H2 within ~25 h duration while its ball milled counterpart containing 5 wt.% n-TiN/n-TiC/n-ZrC desorbs the same quantity of H2 within a slightly shorter duration of about 16 h at 100 °C. The desorption curves clearly indicate that H2 desorption occurs in two stages where the rapid desorption Stage I corresponds to reaction described by Equation (1) and a slow Stage II corresponds to reaction described by Equation (2) [14,16,19]. Furthermore,it has been found [16] that the interstitial compound additives do not substantially affect the apparent activation energy of Stage I dehydrogenation but are able to strongly reduce the apparent activation energy of Stage II dehydrogenation. Accordingly,for thermal dehydrogenation in Stage I the average apparent activation energy,EA,for the interstitial additives is within the range of 87-96 kJ/mol whereas,for comparison,the metallic additives of n-Fe and n-Ni show drastically smaller apparent activation energy on the order of 55-58 kJ/ mol,most likely,due to the LiAlH4 lattice destabilization. The average apparent activation energy for thermal dehydrogenation in Stage II is in the range of 63-80 kJ/mol in the order of EA (n-ZrC) < EA (n-TiN = n-TiC) and is lower than that for the nanometric metal additives n-Ni and n-Fe [16]. We have not observed successful rehydrogenation for LiAlH4 containing 5 wt.% of nanometric n-Ni/n-Fe or n-ZrC/n-TiN/n-TiC additives under the pressure up to 100 bar H2 and temperatures within the range 55-250 °C for several hours which clearly indicates that LiAlH4 with catalytic additives is irreversible. However,it has been found [33,34] that LiAlH4 which was initially ball milled with TiCl and subsequently dehydrogenated to a mixture of (LiH + Al) according to Equation (2),could be partially rehydrogenated back to LiAlH4 at room temperature in low-boiling dimethyl ether under 100 bar H2 pressure.
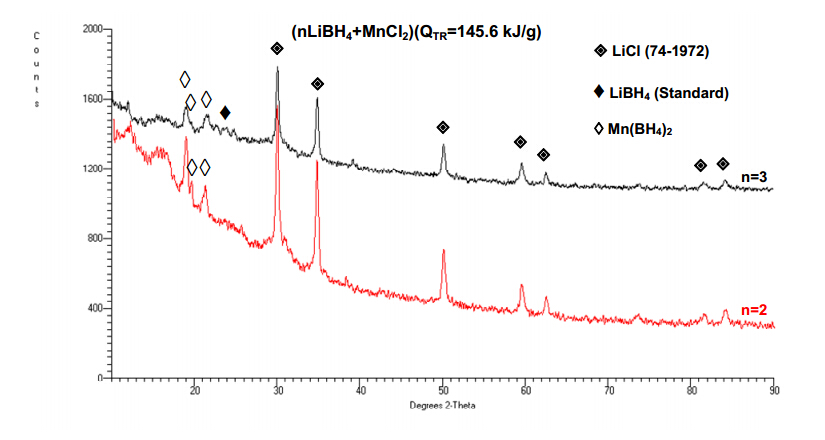
Another hydride recently synthesized in our laboratory is a manganese borohydride,Mn(BH4)2 [37]. Its theoretical H2 capacity is 9.5 wt.%. This borohydride has been successfully synthesized by the mechano-chemical activation synthesis (MCAS) of the (MnCl2 + nLiBH4) mixture,where n a number of moles LiBH4. Measurements of the particle size showed that the average particle size of 7.5 ± 2.6 µm is the smallest one after milling for 2 h with QTR = 145.6 kJ/g. After milling for 5 h with QTR = 364 kJ/g the average particle size increases to 16.1 ± 6.3 µm due to agglomeration of particulate [36]. However,it must be kept in mind that the individual powder particles forming an agglomerate could be much smaller than the average value. Figure 13 shows the XRD pattern for the ball milled n = 2 and 3 nanocomposites after MCAS with the milling energy input QTR = 145.6 kJ/g (a milling mode IMP68-4B-R132-2h). Only the diffraction peaks of LiCl and Mn(BH4)2 are clearly seen. The identification of Mn(BH4)2 was based on the data reported in [36] which show that it has a trigonal/hexagonal lattice structure (the space group P3112) with the lattice parameters a = 10.435(1)Å and c = 10.835(2)Å. The pattern for the n = 3 nanocomposite is nearly identical to the n = 2 nanocomposite with the exception that a remnant very weak and broad LiBH4 peak is barely visible. Nearly identical XRD patterns were observed after milling with a much lower energy input,QTR = 36.4 kJ/g (0.5 h). Based on the XRD and FT-IR analysis [36] it is clear that the synthesis of Mn(BH4)2 occurs according to “metathesis” reaction during MCAS described by:
MnCl2 + nLiBH4→Mn(BH4)2 + 2LiCl + (n-2)LiBH4 (11)
As a result of reaction in Equation (11),simultaneously with Mn(BH4)2 a by-product LiCl is also formed which is just a “dead-weight” for the system reducing the overall H2 capacity. It is quite remarkable that “metathesis” reaction in Equation (11) is able to occur at a small input of milling energy QTR = 36.4 kJ/g. It suggests that a thermodynamic barrier for “metathesis” reaction in a LiBH4/MnCl2 system is very low. The peaks of Mn(BH4)2 and LiCl are broadened that confirm that the synthesized hydride is heavily nanostructured. The calculations of crystallite (grain) size showed that the synthesized Mn(BH4)2 hydride is nanocrystalline exhibiting a crystallite (grain) size within the range from ~21 to ~14 nm which slightly decreases with increasing milling energy input,QTR. The crystallite (grain) size of LiCl is very close to 30 nm regardless of the milling energy input,
QTR [37].
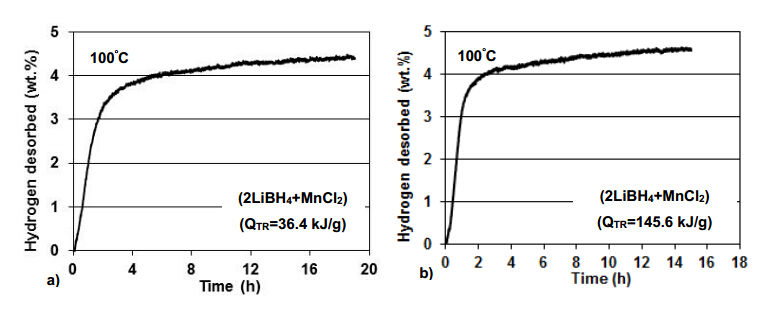
Figure 14 shows the desorption curves at 100 °C for small and medium milling energy inputs during MCAS synthesis. The maximum H2 desorption quantity is very similar for both milling energy inputs and does not exceed ~4.5 wt.%. The maximum theoretical H2 capacity for the n = 2 mixture (2LiBH4 + MnCl2) is 4.76 wt.% (Table 1">Table 1 in Reference [36]). Since the maximum H2 quantity desorbed in Figure 14 is no larger than the maximum theoretical H2 capacity then it is clear that no measurable diborane gas (B2H6) was desorbed as also evidenced by gas mass spectroscopy reported in [36]. XRD patterns after desorption at 100°C did not show peaks of Mn(BH4)2 [36] which means that the synthesized hydride completely decomposed during dehydrogenation according to reaction Equation (12):
Mn(BH4)2®Mn + 2B + 4H2 (12)
No XRD peaks of Mn and B,as would be required by reaction Equation (12),were observed in [36] which strongly confirms that both elements are amorphous after dehydrogenation.
The values of the apparent activation energy for dehydrogenation reported in [37] were very low. The apparent activation energy for the n = 3 nanocomposite decreased monotonically from ~70 to ~59 kJ/mol with increasing milling energy input whereas the apparent activation energy for the n = 2 nanocomposite decreased fromabout 65 kJ/mol for QTR = 36.4 kJ/g to about 53 kJ/mol for QTR = 145.6 kJ/g and then again increased to ~59 kJ/mol for the QTR = 364 kJ/g. These changes closely followed the variations in the average pow der particle size obtained with the varying milling energy input suggesting that for the n = 2 ratio the apparent activation energy seems to somehow depend on the particle size rather than the crystallite (grain) size of Mn(BH4)2. Since the LiCl by product is useless and just reduces the maximum capacity of H2 available from the Mn(BH4)2-LiCl system,it must be removed by chemical methods (Soxhlet extraction) [37].
4. Discussion
In the present comprehensive review two important groups of complex hydride systems for hydrogen generation are being reviewed. In the first group,the hydride systems that are capable of generating H2 through mechanical dehydrogenation phenomenon at the ambient temperature are discussed. There are few diverse systems in this group such as lithium alanate (LiAlH4) with such additives as nanoiron (n-Fe),lithium amide (LiNH2) (a hydride/hydride system),and manganese chloride MnCl2 (a hydride/halide system),as well as another hydride/hydride system consisting of lithium amide (LiNH2) and magnesium hydride (MgH2),and finally,a hydride/halide LiBH4-FeCl2 system. These hydride systems are capable of releasing from ~4 to ~7 wt.% H2 at the ambient temperature during relatively short duration ball milling. The second group encompasses systems that generate H2 at only slightly elevated temperatures (up to 100 °C). In this group,lithium alanate (LiAlH4) ball milled with the nanometric n-Fe and n-TiN/n-TiC/n-ZrC additives is a prominent system that can relatively fast generate up to 7 wt. % H2 at 100°C. The other hydride is manganese borohydride (Mn(BH4)2) synthesized by mechano-chemical activation synthesis (MCAS). In a ball milled nanocomposite co-existing with LiCl,Mn(BH4)2 can desorb ~4.5 wt.% H2 at 100 °C within a reasonable dehydrogenation time.
It is interesting for the readership to discuss shortly some practical application aspects of the hydrides reviewed in the preceding sections. It must be clearly reiterated that the hydride systems for hydrogen generation are not typical,reversible on board,hydrogen “storage” systems in the classical meaning of this word. In contrast to typical “on-board” storage systems,the H2 generating systems,after being exhausted and converted into elemental or other chemical species,would not require reversibility “on-board” but could be chemically regenerated or converted to useful chemical compounds “off-board”,i.e. in a chemical plant.
For example,it may be envisaged that the rapid mechanical dehydrogenation phenomenon occurring at the ambient temperature,discussed earlier in the present review for some hydride/nano-metal/nano-interstitial compound (LiAlH4 + 5 wt.% n-Fe/n-TiN/n-TiC/n-ZrC),hydride/hydride (1LiAlH4 + 1LiNH2) and hydride/halide (3LiAlH4 + MnCl2 and 2LiBH4-FeCl2) systems,could be applied in practical engineering terms for generating large quantities of hydrogen on demand for auxiliary power generation systems coupled with PEM fuel cells and batteries. Further possible practical applications for the rapid hydrogen generators are low power remote fuel cells or portable gas analyzers [41,42],fuel cell off-road vehicles and cordless lawn mowers running with a fuel cell instead of hydrogen combustion engine [43]. The rapid H2 generators can also be used in a number of chemical processes where a continuously reducing atmosphere is needed f or a completion of the chemical process [4]. They could also have an application in a military sector for supplying hydrogen to micro fuel cells in portable devices needed for soldiers on a mission in remote areas [4].
Furthermore,a surprisingly very low mechanical energy input required to generate H2 from several such systems like (1LiAlH4 + 1LiNH2) and (2LiBH4 + FeCl2),without the necessity for applying elevated temperatures,renders the rapid mechanical dehydrogenation phenomenon useful for powering various fuel cell-run devices mentioned above in such a way that the mechanical energy for H2 release could even be provided by human power similarly,for instance,to pedaling a stationary bicycle.
Other hydride/hydride or hydride/halide systems with even more rapid mechanical dehydrogenation rate and higher released H2 capacity,which could practically serve as “rapid mechanical hydrogen generators” should be sought. Several similar high H2 capacity systems are now under investigation in our laboratory and will be reported in further publications.
Acknowledgements
This research was financially supported by a Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery Grant which is gratefully acknowledged.
Conflict of Interest
All authors declare no conflicts of interest in this paper.










 DownLoad:
DownLoad: