1. Introduction
In many sub-Saharan countries, people are not only food insecure, but are also chronically exposed to high levels of mycotoxins in their diet. Mycotoxins are toxic secondary metabolites produced by fungi and contaminate various agricultural commodities either before harvest or under postharvest conditions [1]. Key mycotoxins that are of greatest public health and agro-economic significance are: aflatoxin (AF), ochratoxins (OT), fumonisins (F), zearalenone (ZEN), trichothecenes, ergot alkaloids and tremogenic toxins. The food-borne mycotoxins likely to be of greatest significance in Africa and other tropical developing countries are aflatoxin and fumonisin [2]. Aflatoxin is produced by fungi of the genus Aspergillus. There are three main species of the fungi: Aspergillus flavus, Aspergillus parasiticus and rarely Aspergillus nomius are noted to producing aflatoxin as secondary metabolites in agricultural products prone to fungal infection. Aspergillus species generate four significant types of aflatoxins: B1, B2, G1 and G2. The hierarchy of toxicity are in the order of B1 > G1 > B2 > G2 [3]. At present, aflatoxin B1is considered to be among the strongest natural known carcinogens [4].
According to FAO [5], maize consumption in Africa ranges from 85 kg/year per person in Eastern and Southern Africa to 105 kg/year per person in West Africa. Unfortunately, maize is one of the richest substrates for aflatoxin contamination with even standing crop getting high degree of contamination [6]. Insects, temperature fluctuations and relative humidity in the tropics have been attributed to the acceleration and rapid multiplication of mould and aflatoxin contamination of maize in the tropics [7,8].
Hermetic Storage is based on the principle of generating an oxygen-depleted, carbon dioxide-enriched atmosphere caused by the respiration of living organisms in the sealed storage system [9,10]. The triple-layer hermetic bag (TLHB) technology has proven to be effective in storing a variety of crops against insect pest [11,12,13]. It has been disseminated to millions of farmers in West and Central Africa, with close to 50% of the cowpea not sold at harvest being stored in these simple containers [14].
As a result of its effectiveness against insects, questions concerning its potential against mould damage and mycotoxins contamination especially in regions with extreme temperature fluctuation have come up. A recent laboratory study by Williams et al.concluded that THLB provided excellent protection against mould growth and aflatoxin production across a wide range of grain moisture conditions compared to non-hermetic containers [15]. The current study however, assesses the levels of temperature conditions on the effectiveness of multiple-layer hermetic bag for bio-rational management of aflatoxin in stored maize.
2. Materials and methods
A factorial treatment combination of three temperature levels (16, 30 and 38 °C) and two bagging technologies (triple-layer hermetic bag and polypropylene interwoven bag) were used for the experiment in a completely randomized design. Sample of maize from Obatanpa variety cultivated on farmer’s field was sieved with laboratory test sieve (Impact, United Kingdom) and winnowed to remove chaffs, broken grains, and identified insect (Sitophillus zeamais) present in the grains to get a clean maize sample for storage.
Grains to be stored in non-hermetic bag were sterilized at a temperature of 60 °C for 3 hours to kill Sitophillus zeamais eggs present. A 5-kg capacity high density polyethylene (HDP) hermetic bags (150 μm thick and measure 34 cm × 62 cm in width and length, respectively) supplied by Bioplastic Ghana and polypropylene bags bought from the market were filled with 2.5 kg grains and gently pressed to evacuate all air present and tied quickly with cotton rope to prevent the re-entry of air.
The bagged samples were grouped into three batches representing the experimental treatments: low temperature (16 °C), room temperature (30 °C), and warm temperature (38 °C). The first batch of maize samples was stored in an incubator in the Entomology Laboratory, Crop Science Department, School of Agriculture, and College of Basic and Applied Sciences (CBAS), University of Ghana at temperature of 16 °C. The second batch of maize sample was stored at an average room temperature of 30 °C at the Africa Regional Postgraduate Programme in Insects Science (ARPPIS)-West Africa Center, University of Ghana, Legon. The last batch of maize sample was also stored in separate room at a controlled warm temperature of 38 °C in ARPPIS University of Ghana, Legon.
Each treatment was replicated three times and stored for three months. Changes in relative humidity, moisture content, oxygen and carbon dioxide were monitored and recorded during the period of storage. Sampling of maize for aflatoxin analysis was done before storage and three months after storage.
2.1. Determination of oxygen (O2) and carbon dioxide (CO2)
Prior to the opening of the triple-layer hermetic bags, oxygen and carbon dioxide levels were measured with the aid of GrainPro oxygen analyser (SCY-2A, MAOAN, O2 ≤ ±1.0 and CO2 ≤ ±3.0), butterfly needle and epoxy glue. The instrument, calibrated in nitrogen gas to 21% was used to measure the oxygen level in the hermetic bags. To take measurements, the inner HDPE lining was punctured with the analyser needle at the top, middle and bottom. All punctures on the bag walls were sealed with epoxy glue. Subsequent measurement was performed from the same spot by simply opening and closing the lid of the butterfly needle. This was done immediately after the set up and repeated daily.
2.2. Isolation and identification of fungal pathogens
Isolation and identification of fungal pathogens from the samples was carried out in Pathology Laboratory, Crop Science Department in the School of Agriculture, CBAS, Legon on potato dextrose agar (PDA) before and after storage. Potato dextrose agar was prepared by dissolving 3.9 g of PDA powder in 100 mL of distilled water in a 250 mL conical flask. The conical flask together with the content was carefully swirled, covered with aluminum foil and autoclaved at 1.05 kg/cm2 pressure and 121 °C for 15 minutes. To inhibit bacterial growth, 10 mg of amoxicillin was added to the PDA. The prepared PDA was poured in 9 cm sterilized petri dishes and allowed to solidify. Surface sterilization of well mixed, random sampled maize was done in 1% sodium hypochlorite for 30 seconds, blotted dry with filter paper and the sterilized grains placed at a rate of 10 grains per petri dish on cooled potato dextrose agar. The morphological identification of fungus associated with maize grains was done by scraping mycelia plugs advancing from margins of the grains with flamed scalpel. The plugs were mounted on slides for microscopic examination using distilled water. The prepared slides were examined and identification of the isolates was done based on colour, morphology of mycelial, conidia and sporulating structures as described by Agrios [16], Barnett and Hunter [17].
2.3. Sampling procedure for initial and final levels of aflatoxin in maize
Maize transported to the experimental site was sun dried to moisture content of between 12-13% and thoroughly mixed in a container to obtain a uniform sample. Four composite samples, each weighing 2 kg was taken from the uniformly mixed sample, by sub-sampling from the different parts of the container to obtain the initial samples for aflatoxin analysis. Subsequentmaize samples from either storage bag technology were also obtained by thoroughly mixing and drawing of 1 kg subsample from the 2.5 kg sample for aflatoxin analysis. The initial and final aflatoxin content of maize was determined at the Mycotoxins and Histamine Laboratory of Ghana Standard Authority, Accra, Ghana.
2.4. Determination of aflatoxin types in stored maize
The aflatoxin levels were determined according to ISO 16050 test methods [18]. The 1-kg sub-sampled maize was ground with laboratory mill (IKA-Werke, IKA, and MF10B) and blended and mixed thoroughly before sampling into sample containers. Two grams (2 g) of sodium chloride and 100 mL of extraction solvent were added to 20 g of milled maize. The solution was shaken and homoginised for 3 minutes. The extract was filtered using a paper filter and 20 mL of the filtrate was diluted with 60 mL of Phosphate-buffered saline (PBS), mixed well and filtered again using glass microfiber filter. Purification was carried out by passing the extract through immunoaffinity column (Romer Labs, Austria) containing antibodies specific for aflatoxins B1, B2, G1 and G2. The aflatoxin was eluted with 1.0 mL of methanol and quantified by reverse-phase High Performance Liquid Chromatography (HPLC) with spectrofluorometric detector (RF-20A, Shimadzu Corporation, Japan). Aflatoxin was derivatised in a post-column reaction chamber (Kobra cell) by adding potassium bromide of 0.119 g to 1 L of the mobile phase followed by fluorescence detection. Aflatoxin is identified by comparing the retention time of the peak detected in the chromatogram of the test solution with the retention time of the peaks of the standard for aflatoxins.
2.5. Data analysis
All data were subjected to Analysis of Variance procedure in GENSTAT statistical package (version 12) and Microsoft Excel 4.0. Least Significant Difference (LSD) was used to separate the means when there were significance differences.
3. Results
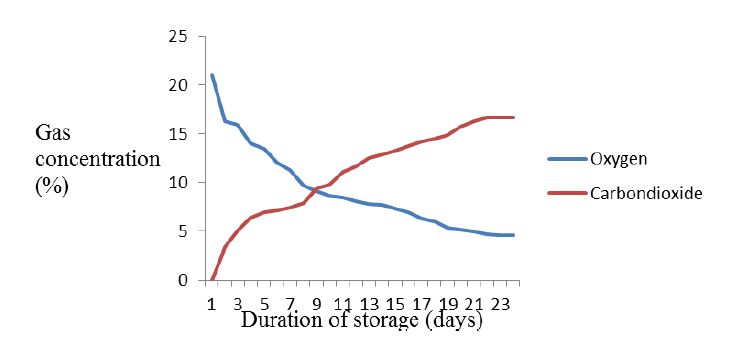
3.1. Changes in oxygen and carbon dioxide concentration in triple-layer hermetic bag
Changes in atmospheric gas content of maize stored in triple-layer hermetic bag are shown in Figure 1. The initial oxygen level of 21% dropped significantly to 13.5% after fifth day of storage. However, the level of oxygen depletion and carbon dioxide elevation remained relatively constant after 20 days of storage in the hermetic bags regardless of the temperature levels under TLHBs were kept. The lowest concentrations of oxygen and highest carbon dioxide of 4.6 and 16.7% were recorded respectively after 23 days maize storage in the triple-layer hermetic bags.
3.2. Effect of moisture content and relative humidity on accumulation of aflatoxin at different temperature levels
The analysis of variance showed significant differences (p < 0.05) in moisture content and accumulation of aflatoxin under different temperature conditions in relation to the storage bag technologies (Tables 1-4 and Figure 2). The initial mean moisture content of 12.3% increased to 14.4% after three months of storage of grain in hermetic bag under low temperature condition (16 °C). On the contrary, the initial moisture content reduced significantly to 9.9% after three months of storing maize grains in polypropylene bag at 38 °C. The relative humidity of each temperature level remained relatively stable during the experiment. The lowest (45.7%) and highest (78.9%) mean relative humidity were recorded at temperatures 38 and 16 °C respectively. An optimum level of relative humidity (66%) was recorded at room temperature.
Table 1.Effect of temperature and moisture content on levels of aflatoxin G1 after three months storage.
| Bag type | Temperature (°C) | Initial-MC (%) | Initial-AFG1 (μg kg-1) | Month-3 MC (%) | Month-3AFG1 (μg kg-1) |
| TLH Bag | 16 | 12.3a | ND | 14.4a | ND |
| 30 | 12.3a | ND | 12.2a | ND |
| 38 | 12.3a | ND | 11.8b | ND |
| Polypropylene Bag | 16 | 12.3a | ND | 13.9b | ND |
| 30 | 12.3a | ND | 11.5b | ND |
| 38 | 12.3a | ND | 9.9c | ND |
| a-c: Values in the same column not sharing a common subscript are significantly different at LSD (p < 0.05); TLH = triple-layer hermetic, ND = Not Detected |
Table 2.Effect of temperature and moisture content on levels of aflatoxin G2 after three months storage.
| Bag type | Temperature (°C) | Initial-MC (%) | Initial-AFG2 (μg kg-1) | Month-3 MC (%) | Month-3 AFG2 (μg kg-1) |
| TLH Bag | 16 | 12.3a | ND | 14.4a | ND |
| 30 | 12.3a | ND | 12.2a | ND |
| 38 | 12.3a | ND | 11.8b | ND |
| Polypropylene Bag | 16 | 12.3a | ND | 13.9b | ND |
| 30 | 12.3a | ND | 11.5b | 0.6 |
| 38 | 12.3a | ND | 9.9c | ND |
| a-c: Values in the same column not sharing a common subscript are significantly different at LSD (p < 0.05); TLH = triple-layer hermetic, ND = Not Detected |
Table 3.Effect of temperature and moisture content on levels of aflatoxin B2 after three months storage.
| Bag type | Temperature (°C) | Initial MC (%) | Initial-AFB2 (μg kg-1) | Month-3 MC (%) | Month-3 AFB2 (μg kg-1) |
| TLH Bag | 16 | 12.3a | 4.2a | 14.4a | 14.7a |
| 30 | 12.3a | 4.2a | 12.2a | 5.8c |
| 38 | 12.3a | 4.2a | 11.8b | 4.7c |
| Polypropylene Bag | 16 | 12.3a | 4.2a | 13.9b | 9.3b |
| 30 | 12.3a | 4.2a | 11.5b | 8.2b |
| 38 | 12.3a | 4.2a | 9.9c | 4.2c |
| a-c:Values in the same column not sharing a common subscript are significantly different at LSD (p < 0.05); TLH = triple-layer hermetic |
Table 4.Effect of temperature and moisture content on levels of aflatoxin B1 after three months storage.
| Bag type | Temperature (°C) | Initial MC (%) | Initial-AFB1 (μg kg-1) | Month-3 MC (%) | Month-3 AFB1 (μg kg-1) |
| TLH Bag | 16 | 12.3a | 34.0a | 14.4a | 71.6a |
| 30 | 12.3a | 34.0a | 12.2a | 44.3d |
| 38 | 12.3a | 34.0a | 11.8b | 39.2e |
| Polypropylene Bag | 16 | 12.3a | 34.0a | 13.9b | 67.8b |
| 30 | 12.3a | 34.0a | 11.5b | 47.7c |
| 38 | 12.3a | 34.0a | 9.9c | 34.6f |
| a-f: Values in the same column not sharing a common subscript are significantly different at LSD (p < 0.05); TLH = triple-layer hermetic. |
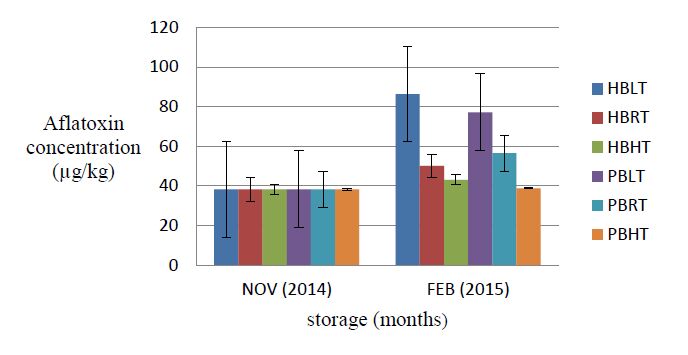
Aflatoxin analysis showed no contamination of maize grains with AFG1 over the three months period in triple-layer hermetic and polypropylene bag. Similarly, the stored maize sample was found to contain no AFG2 at beginning of storage. This trend however changed at the end of storage following the accumulation of mean Aflatoxin G2 of 0.6 μg kg−1in maize samples stored in polypropylene bag at 30 °C. Also, among all the various treatments, maize sample stored in hermetic bag at 16 °C accumulated the highest level of aflatoxin B2 over the period of storage. Furthermore, significant differences (p < 0.05) were observed in AFB1contamination levels irrespective of the kind of temperature treatment received by the maize.
Generally, as moisture content of grain increased, the level of aflatoxin contamination also increased in the different storage bag technologies used. This trend was profoundly evident when AFB1 in hermetic bag increased considerably from the initial 34.0 to 71.6 μg kg−1within three months of storage under 16 °C following an increase of grain moisture content from 12.3 to 14.4%. Conversely, as moisture content dropped from the initial level of 12.3 to 9.9% in the polypropylene bag at 38 °C, the lowest level of aflatoxin B1 (34.6 μg kg−1) was recorded.
3.3. Fungi identified in maize samples
The experiment showed that all maize grains used for the study were previously infected with the aflatoxigenic fungi: Aspergillus flavus. In addition to Aspergillus flavus, other common fungi such as Aspergillus niger and Rhizopus stolonifier were observed in aflatoxin contaminated maize after storage as shown in Figure 3. Micrographs of all the three fungi identified under the microscope are presented in Figure 4.
4. Discussion
Effect of temperature levels on aflatoxin contamination of maize stored in triple layer hermetic bag and polypropylene interwoven bag
Aflatoxin is considered by US Food and Drug Administration (FDA) to be unavoidable contaminant of food because factors contributing to its production are usually beyond human control [19,20]. Temperature, moisture content and relative humidity are the three key climatic factors that influence growth of moulds and fungi [21]. Results of grain storage in the tropics have revealed a direct relationship between temperature, moisture content and relative humidity, that is, as temperature increases; grain will lose moisture to the surrounding air, thereby increasing the relative humidity [22]. Hence, inappropriate drying and reabsorption of moisture by previously dried maize (≤ 14) would predispose the grain to aflatoxin contamination [23]. The current research revealed that, different levels of temperature have significant impact on the percentage moisture acquired by grains and the relative humidity of the storage environment in relation to different storage bag technologies. Maize grains stored in triple-layer hermetic and polypropylene interwoven bags had an average initial moisture content of 12.3%. This changed steadily after three months with respective to the different levels of temperature and type of storage bag. The good permeability of warm air into the polypropylene interwoven bags might have resulted in moisture level below the average initial moisture content. This was consistent with other studies which concluded that, the minimum restriction offered by the polypropylene bag to air, created conditions for proper air circulation and subsequent reduction in grain moisture content [24,25]. Well-dried grains in the polypropylene bags did not support mould growth and aflatoxin production since free water according to Hell et al. is necessary for aflatoxigenic fungi to contaminate grains with aflatoxin [1]. The lowest aflatoxin level recorded in the polypropylene interwoven bag at 38 °C might also be due the low relative humidity (45.7%) recorded in the storage environment. Relative humidity of below 65% decreases the population density of aflatoxigenic fungi [26]. The interactions between moisture and relative humidity have been reported to have significant influence on the level of aflatoxin accumulation in storage. Moisture content below 10% and relative humidity below 70% are inimical to destructive biotic agents such as fungi, insects, mites, etc. [27].
There was a general rise in moisture content of maize grain stored in the hermetic and polypropylene interwoven at 16 °C. The rise in moisture content within the bags was as result of metabolic activities of grains and lower temperature condition in the storage environment (incubator). This phenomenon might have resulted in the generation of condensed water on the walls of the bag. Condensed water wets grains close to the walls and the bottom of the bags to increase the moisture content of grains creating a more favourable environment for aflatoxin production by fungi present in the grain prior to storage [27]. An average high relative humidity (78.9%) in the storage environment coupled with high moisture content of grains within the different bag technologies created suitable condition for the proliferation and contamination of maize by fungi present in stored maize [28].
The change in aflatoxin levels from 38.2 to 50.1 μg kg−1 in the hermetic bag and 38.2 to 56.2 μg kg−1 in the polypropylene bag at room temperature may be due to the stable environmental conditions within the store room. This validates the report by Ansah, that when environmental conditions are stable within storage structures, the initial levels of aflatoxin concentration could be contained in the triple-layer hermetic bag and polypropylene interwoven bag [29].
The differences in aflatoxin accumulation in the two storage bag technologies at room temperature may be due to amount of oxygen and carbon dioxide present in each of them. This also concurs with the study by Sudini that, the free atmospheric air couple with the presence of aflatoxigenic fungi in cloth bags accounted for the higher level of aflatoxin in cloth bag than triple-layer bag in peanut stored at room temperature [30]. It is worthy to point out that, the minimum amount of oxygen gas (4.6%) recorded in the triple-layer hermetic bag was capable of killing all insects present, which confirms previous studies by Anankware et al. [13]. But unlike insects, many storage fungi are capable of growing at low pressure of oxygen and reduction of oxygen is often not sufficient to prevent moulding [31]. Decreasing oxygen to < 0.14% and elevating carbon dioxide to > 50% is required for complete inhibition of growth and subsequent aflatoxin contamination by aflatoxigenic fungi [32]. The percentage change (31.2%) of aflatoxin accumulated after three months of storage in the hermetic bag at room temperature was within the acceptable limit of 15 μg kg−1 set by the Codex Alimentarius Commission and the Ghana Standard Authority [33].
5. Conclusion and recommendation
The study had proven that different levels of temperature have the capacity to dictate the amount of moisture and relative humidity in triple-layer hermetic bag and subsequent quantity of aflatoxin accumulated overtime. The triple-layer hermetic bag and polypropylene interwoven bag cannot prevent further contamination of aflatoxin but, can only limit the amount produced provided the grains are dried at safe moisture content of ≤ 12%. Further studies need to be carried out to ascertain the cause of the significant reduction in aflatoxin in maize kept in stable warm temperature conditions and its impact on major quality parameters of maize.
Acknowledgement
The authors wish to acknowledge Bioplastic Ghana, Accra, Mycotoxin and Histamine Laboratory of Ghana Standard Authority, Accra, School of Agriculture, and University of Ghana for their diverse supports.
Conflict of interest
The authors hereby declare that the work was not financed by any company. It formed part of the corresponding author’s Master of Philosophy research work.









 DownLoad:
DownLoad: