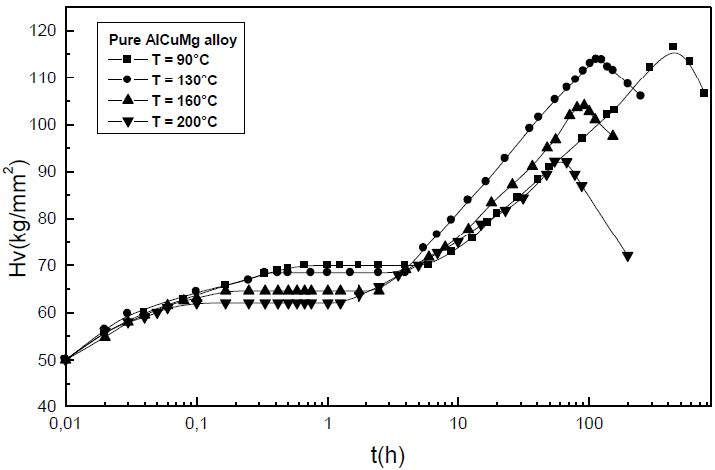
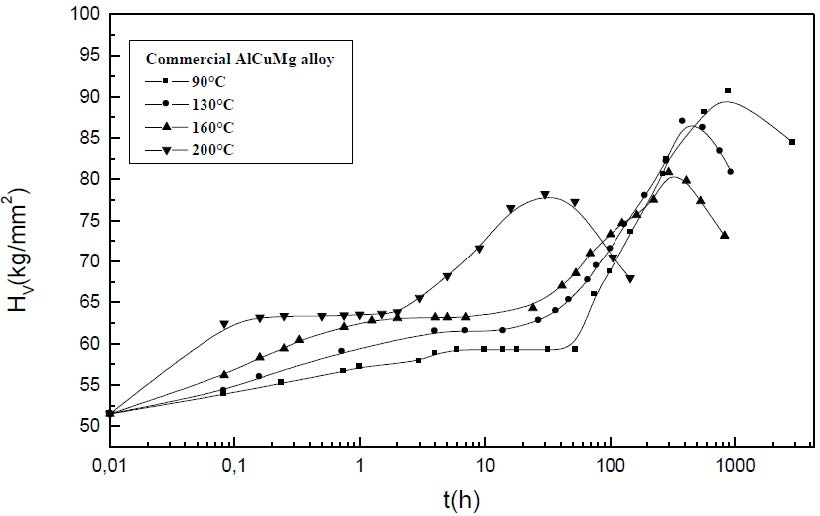
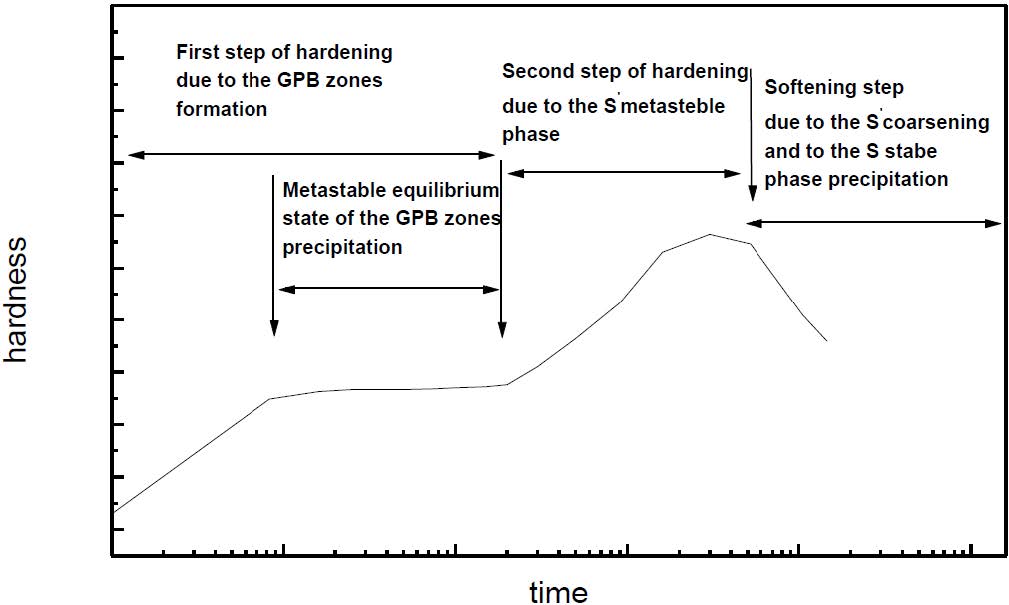
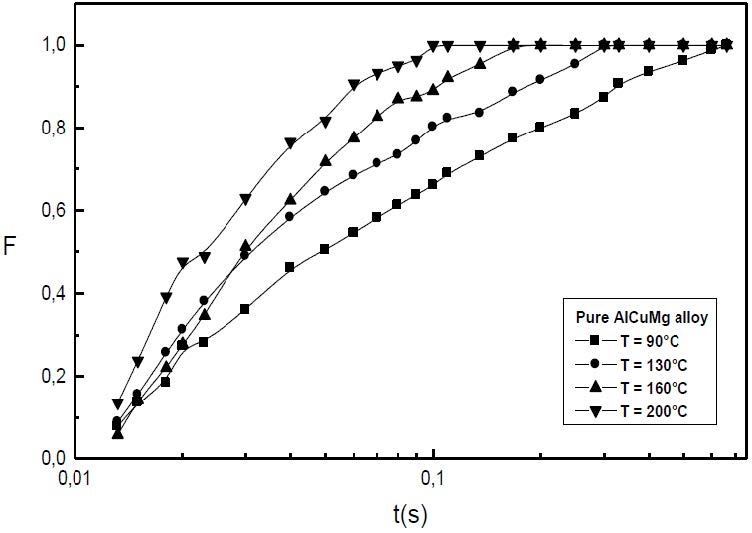
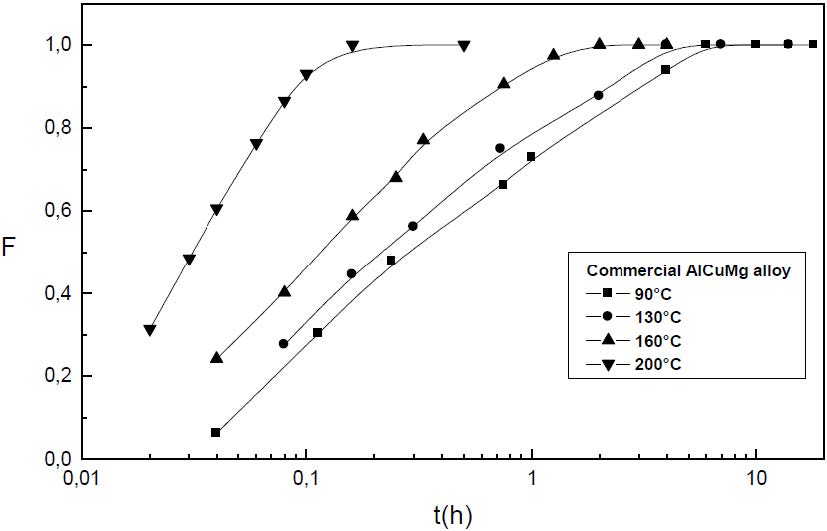
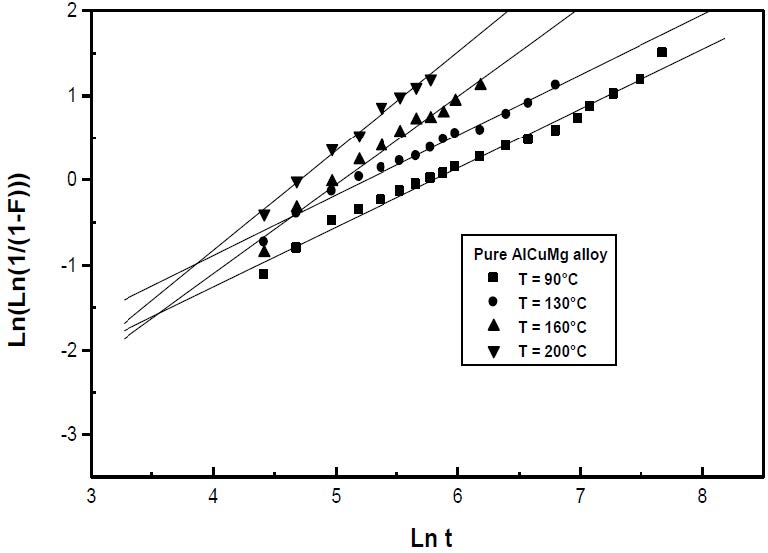
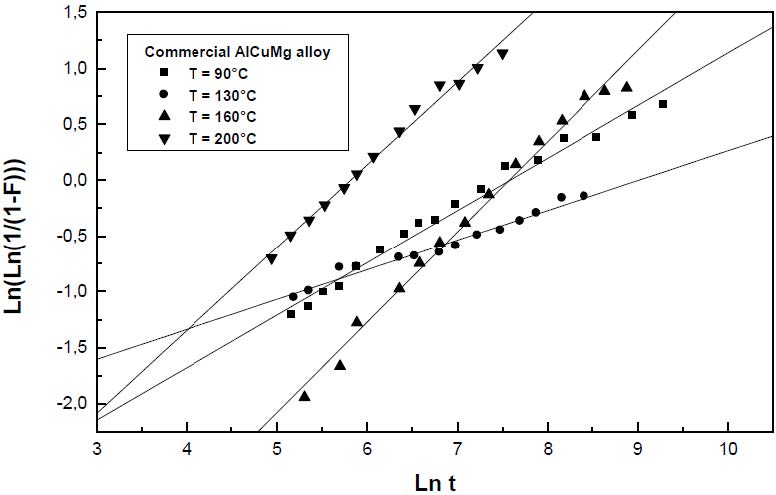
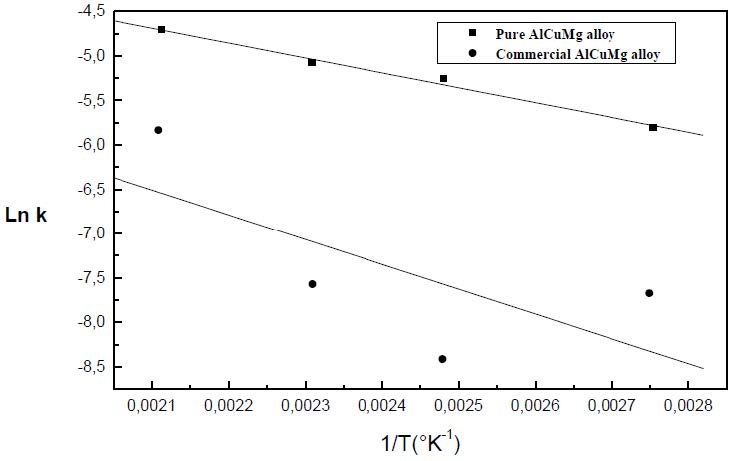
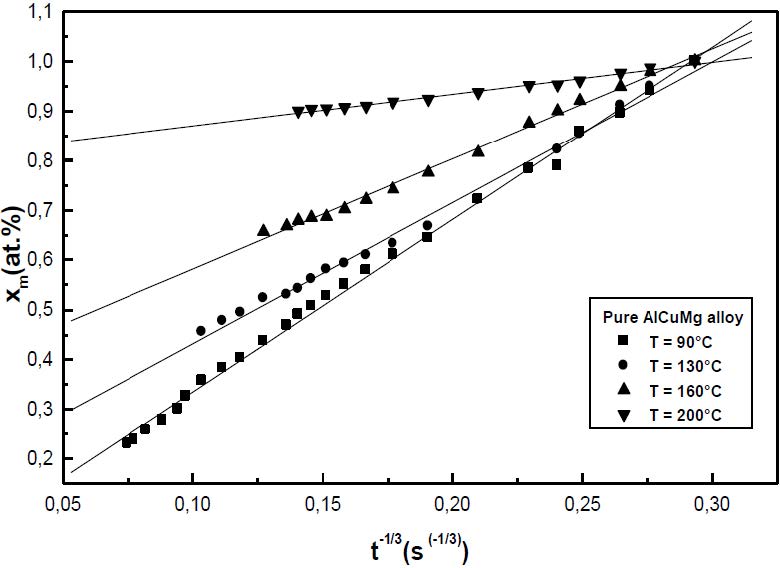
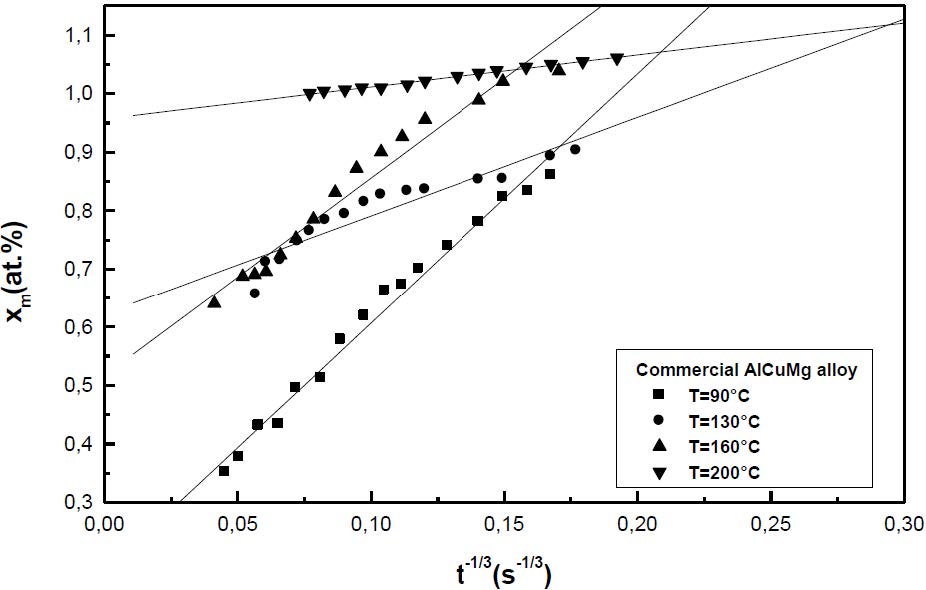
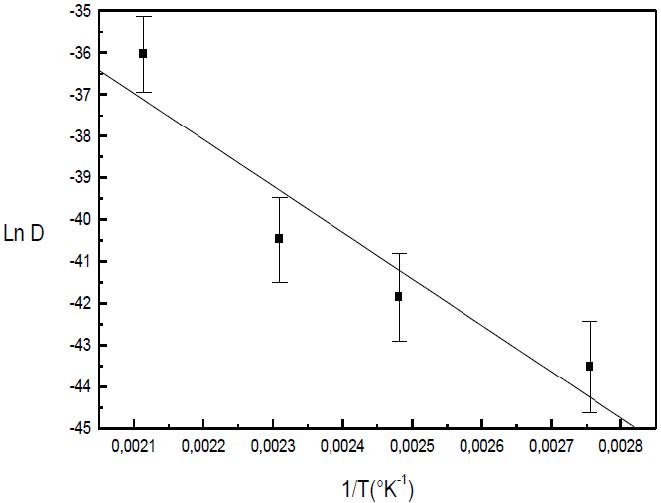
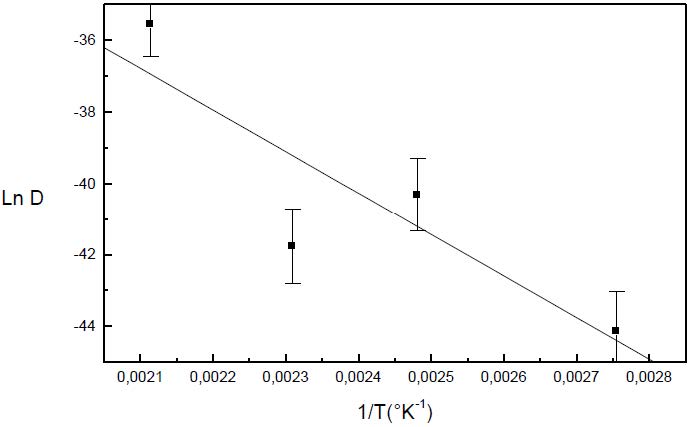
The formation of the Guinier-Preston-Bagaryatsky zones in Al-Cu-Mg, controlled by the solute atoms diffusion, occurs through a nucleation, growth and coarsening phenomenon. Both growth and coarsening regime are well described, respectively, by the JMAK model of growth and the LSW theory. In the commercial Al-Cu-Mg alloy, the presence of Fe and Si atoms leads to the formation of soluble particles such Al2Cu and Mg2Si, and insoluble particles such Al12Fe3Si, Al7Cu2Fe and Al6(Fe, Cu) during heat treatment. Then, some of the Cu and Mg atoms are removed from the solid solution and the effective solute atom concentration in the matrix during the heat treatment is reduced leading a reduction in the driving force of the GPB nucleation and growth and a slowing down the nucleation growth reaction .The diffusion coefficient of the solute atom in the alloy, in both pure Al-Cu-Mg and commercial Al-Cu-Mg alloys, are determined during the GPB zones coarsening. No significative difference exists between the diffusion coefficient of the solute atoms in the pure and in the commercial Al-Cu-Mg alloys during the GPB zones coarsening because some of the excess vacancies are eliminated at the sinks and the driving force of the coarsening reaction is due only to the interfacial energy.
1.
Introduction and main results
Our goal of this paper is to consider the existence of nodal solution and ground state solution for the following fractional Kirchhoff equation:
where a,b,k are positive real numbers. Let α∈(34,1) such that 2∗α=63−2α∈(4,6) and V(x)∈C(R3,R+), Ω⊂R3 is a bounded domain with a smooth boundary ∂Ω. ‖u‖2K:=∫R3∫R3|u(x)−u(y)|2K(x−y)dxdy. The non-local integrodifferential operator LK is defined as follows:
the kernel K:R3→(0,∞) is a function with the properties that
(ⅰ) mK∈L1(R3), where m(x)=min{|x|2,1};
(ⅱ) there exists λ>0 such that K(x)≥λ|x|−(3+2α) for any x∈R3∖{0};
(ⅲ) K(x)=K(−x) for any x∈R3∖{0}.
We note that when K(x)=1|x|3+2α, the integrodifferential operator LK is the fractional Laplacian operator (−Δ)α:
and in this case
where C(α)=(∫R31−cosξ1|ξ|3+2αdξ)−1 is the normalized constant.
When a=1, b=0 and K(x)=1|x|3+2α, the fractional Kirchhoff equation is reduced to the undermentioned fractional nonlocal problem
Equation (2) is derived from the fractional Schrödinger equation and the nonlinearity f(x,u) represents the particles interacting with each other. On the other hand, recently a great attention has been given to the so called fractional Kirchhoff equation (see [3,10] etc., ):
where Ω⊂RN is a bounded domain or Ω=RN, a>0,b>0 and u satisfies some boundary conditions. Problem (3) is called to the stationary state of the fractional Kirchhoff equation
As a special significant case, the nonlocal aspect of the tension arises from nonlocal measurements of the fractional length of the string. For more mathematical and physical background on Schrödinger-Kirchhoff type problems, we refer the readers to [6] and the references therein.
In the remarkable work of Caffarelli and Silvestre [2], the authors express this nonlocal operator (−Δ)α as a Dirichlet-Neumann map for a certain elliptic boundary value problem with local differential operators defined on the upper half space. This technique is a valid tool to deal with the equations involving fractional operator in the respects of regularity and variational methods. However, we do not know whether the Caffarelli-Silvestre extension method can be applied to the general integro-differential operator LK. So the nonlocal feature of the integro-differential operator brings some difficulties to applications of variational method to problem (1). Some of these additional difficulties were overcome in [12,13]. More studies on this integro-differential operator equation, for examples, positive, negative, sign-changing or ground state solutions, we refer the papers [4,5,9,16] and their references. In [1], the authors considered the obstacle problems for LK about the higher regularity of free boundaries. The obstacle problems for nonlinear integro-differential operators and applications are also available in papers [7,8]. The appearance of nonlocal term not only makes it playing an important role in many physical applications, but also brings some difficulties and challenges in mathematical analysis. This fact makes the study of fractional Kirchhoff equation or similar problems particularly interesting. A lot of interesting results on the existence of nonlocal problems were obtained recently, specially on the existence of positive solutions, multiple solutions, ground states and semiclassical states, for examples, we refer [3,15,19] and the cited references.
In past few years, some researchers began to search for nodal solutions of Schrödinger type equation with critical growth nonlinearity and have got some interesting results. For example, Zhang [20] considered the following Schrödinger-Poisson system:
where p∈(4,6), k(x) and a(x) are nonnegative functions. By using variational method, a ground state solution and a nodal solution for problem (5) were obtained.
Wang [17] studies the following Kirchhoff-type equation:
where Ω⊂R3 is a bounded domain, λ,a,b>0 are fixed parameters, and f(x,⋅) is continuously differentiable for a.e. x∈Ω. By combining constrained variational method with the degree theory, the existence of ground state and nodal solutions for above problem were obtained.
However, as for fractional Kirchhoff types equation, to the best of our knowledge, few results involved the existence and asymptotic behavior of ground state and nodal solutions in case of critical growth. If k(x)≡1, the method used in [20] seems not valid for problem (1), because their result depends on the case k∈Lp(R3)∩L∞(R3) for some p∈(1,2∗α). We employ minimization arguments on suitable nodal Nehari manifold Mk to construct a nodal solution by using various constrained method and qualitative deformation lemma given in [14].
It's worth noting that, the Brouwer degree method used in [18] strictly depends on the nonlinearity f∈C1(R3×R,R), so we have to find new tricks to solve our modeling where we only allow f:R3×R→R is a Carathéodory function. On the other hand, in our modeling, both of the nonlocal fractional operator LK and nonlocal terms ∫R3∫R3|u(x)−u(y)|2K(x−y)dxdy appear, we need to overcome the difficulties caused by the nonlocal terms under a suitable variational framework. In brief, our discussion is based on the standard nodal Nehari manifold method used in [14].
Throughout this paper, we let
where the space X introduced by Servadei and Valdinoci ([12,13]) denotes the linear space of Lebesgue measurable functions u:R3→R such that its restriction to Ω u|Ω∈L2(Ω) and
with the following norm
where Q=(R3×R3)∖(Ωc×Ωc). Then, E is a Hilbert space with inner product
and the norm ‖⋅‖ defined by
X0={u∈X:u=0a.e.inR3∖Ω} is a Hilbert space with inner product (u,v)K=12∫R3∫R3(u(x)−u(y))(v(x)−v(y))K(x−y)dxdy and the Gagliardo norm ‖u‖2K:=(u,u)K=12∫R3∫R3|u(x)−u(y)|2K(x−y)dxdy. In X0 the norms ‖⋅‖K and ‖⋅‖X are equivalent. Thus due to V(x)≥0 in Ω, the inner product ⟨⋅,⋅⟩ and the norm ‖⋅‖ defined above still make sense for the Hilbert space E. Moreover, ‖u‖2K≤a‖u‖2. For more details, we refer to [12].
The following result for the space X0 will be used repeatedly.
Lemma 1.1. ([12]) Let s∈(0,1), N>2s, Ω be an open bounded set of RN, X0↪↪Lp(Ω) for 2<p<2∗α, and X0↪L2∗α(Ω) is continuous. Then, E⊂X0 has the same embedding properties.
A weak solution u∈E of (1) is defined to satisfy the following equation
for any v∈E, i.e.
As for the function f, we assume f:Ω×R→R is a Carathéodory function and satisfing the following hypotheses:
(f1): f(x,t)⋅t>0 for t≠0,∀x∈Ω and limt→0f(x,t)t=0forx∈Ωuniformly;
(f2): There exists q∈(4,2∗α) such that limt→∞f(x,t)tq−1=0 for all t∈R∖{0} and for x∈Ω uniformly;
(f3): f(x,t)|t|3 is an nondecreasing function with respect to t in (−∞,0) and (0,+∞) for a.e. x∈Ω.
Remark 1. We note that under the conditions (f1)-(f3), it is easy to see the function f(x,t)=t3 is an example satisfying all conditions (f1)-(f3).
The main results can be stated as follows.
Theorem 1.2. Suppose that (f1)-(f3) are satisfied. Then, there exists k⋆>0 such that for all k≥k⋆, the problem (1) has a ground state nodal solution uk.
Remark 2. The ground state nodal solution uk with u±k≠0 is a solution of (1) satisfying
where Mk is defined by (7) in next section. For u∈E, u± is defined by
We recall that the nodal set of a continuous function u:R3→R is the set u−1(0). Every connected component of R3∖u−1(0) is called a nodal domain. For this equation talking about the nodal domain of uk in somewhat is difficult for us, we leave it as future topics.
Theorem 1.3. Suppose that (f1)-(f3) are satisfied. Then, there exists k⋆⋆>0 such that for all k≥k⋆⋆, the c∗=infu∈NkJk(u)>0 is achieved by vk, which is a ground state solution of (1) and
where Nk={u∈E∖ {0}|⟨J′k(u),u⟩=0}, uk is the ground state nodal solution obtained in Theorem 1.1.
Comparing with the literature, the above two results can be regarded as a supplementary of those in [3,4,14,17].
The remainder of this paper is organized as follows. In Section 2, we give some useful preliminaries. In Section 3, we study the existence of ground state and nodal solutions of (1) and we prove Theorems 1.1-1.2.
2.
Some technical lemmas
We define the energy functional associated with equation (1) as follows:
According to our assumptions on f(x,t), Jk(u) belongs to C1(E,R) (see [10,12]), then by direct computations, we have
Note that, since (1) involves pure critical nonlinearity |u|2∗α−2u, it will prevent us using the standard arguments as in [14]. Hence, we need some tricks to overcome the lack of compactness of E↪L2∗α(R3).
For fixed u∈E with u±≠0, the function φu:[0,∞)×[0,∞)→R is well defined by φu(s,t)=Jk(su++tu−). We set
The argument generally used is to modify the method developed in [11]. The nodal Nehari manifold is defined by
which is a subset of the Nehari manifold Nk and contains all nodal solutions of (1). Then, one needs to show the minimizer u∈Mk is a critical point of Jk. Because the problem (1) contains a nonlocal term ‖u‖2K, the corresponding functional Jk does not have the decomposition
it brings difficulties to construct a nodal solution.
The following result describes the shape of the nodal Nehari manifold Mk.
Lemma 2.1. Assume that (f1)-(f3) are satisfied, if u∈E with u±≠0, then there is a unique pair (su,tu)∈(0,∞)×(0,∞) such that suu++tuu−∈Mk, which is also the unique maximum point of φu on [0,∞)×[0,∞). Furthermore, if ⟨J′k(u),u±⟩≤0, then 0<su,tu≤1.
Proof. From (f1) and (f2), for any ε>0, there is Cε>0 satisfying
By above equality and Sobolev's embedding theorems, we have
By choosing ε>0 small enough, we can deduce H(s,t)>0 for 0<s≪1 and all t≥0. Similarly,
for 0<t≪1 and all s≥0. We denote D(u)=⟨u+,u−⟩=a(u+,u−)K=−a2∫R3∫R3[u+(x)u−(y)+u−(x)u+(y)]K(x−y)dxdy≥0. Hence, by choosing δ1>0 small, we have
For any δ2>δ1, by condition (f1), it is easy to see
Similarly, we have
By choosing δ2≫1, we deduce
for all s,t∈[δ1,δ2].
Following (11) and (12), we can use Miranda's Theorem (see Lemma 2.4 in [17]) to get a positive pair (su,tu)∈(0,∞)×(0,∞) such that suu++tuu−∈Mk. Similar to the standard argument in [17], we can prove the pair (su,tu) is unique maximum point of φu on [0,+∞)×[0,+∞).
Lastly, we will prove that 0<su,tu≤1 when ⟨J′k(u),u±⟩≤0. Following from su≥tu>0, by a direct computation, we have
On the other hand, ⟨J′k(u),u+⟩≤0 implies that
From (13) - (14), we can see that
So we have su≤1, which implies that 0<su,tu≤1.
Lemma 2.2. There exists k⋆>0 such that for all k≥k⋆, the infimum ck=infu∈MkJk(u) is achieved.
Proof. For any u∈Mk, in view of the definitions of Mk, it follows that
Hence, in view of (8), we have
By choosing ε>0 small enough, we can deduce
for some ρ>0. From assumption (f3), we have
and f(x,t)t−4F(x,t) is nondecreasing in (0,+∞) and non-increasing in (−∞,0) with respect to the variable t. Hence, combining with ⟨J′k(u),u⟩=0, we have
So ck=infu∈MkJk(u)≥0 is well defined.
Let u∈E with u±≠0 be fixed. According to Lemma 2.1, for each k>0, there exists sk,tk>0 such that sku++tku−∈Mk. Hence, by (f1) and the Sobolev's embedding theorem, we have
which implies (sk,tk) is bounded and furthermore by taking any sequence (skn,tkn)→(s0,t0) as kn→∞, we claim that s0=t0=0. In fact, if s0>0 or t0>0. Thanks to sknu++tknu−∈Mkn, we get
Because kn→∞ and {sknu++tknu−} is bounded in E, we have a contradiction with the equality (17). On the other hand, we have
so limk→∞ck=0. By the definition of ck, we can find a sequence {un}⊂Mk satisfing limn→∞Jk(un)=ck, which converges to uk=u++u−∈E. By standard arguments, we have
Denote β:=s3(S)32s, where
Sobolev embedding theorem insures that β>0. So that there exists k⋆>0 such that ck<β for all k≥k⋆. Fix k≥k⋆, in view of Lemma 2.1, we have Jk(su+n+tu−n)≤Jk(un).
By u±n⇀u±inE, we have
By taking n→∞ in both sides of above equality, there holds
On the other hand, by (8) we have
Then,
where |∗|2 and |∗|2∗α are the norm in L2(R3) and L2∗α(R3) repeatedly. So, Fatou's Lemma follows that
where
From the inequality above, we deduce that
We next prove u±≠0. Because the claim u−≠0 is similar, so we only prove u+≠0. Indirectly, we suppose that u+=0 and so A1≥ρ from (15). By letting t=0 in (18), the case B1=0 is done. So we only study the case B1>0 at length. In this case, by the definition of S, we deduce
It happens that,
The inequality (18) and ck<β follows that
which is a contradiction. Thus u+≠0 are claimed.
Then, we consider the key point to the proof of Theorem 1.1, that is B1=B2=0 and then ck is achieved by uk=u++u−∈Mk.
Similarly, we only prove B1=0. Indirectly, we suppose that B1>0. We have two cases.
Case 1: B2>0. Let ˉs and ˉt be the numbers such that
Since φu is continuous, we have (su,tu)∈[0,ˉs]×[0,ˉt] satisfying
If t>0 small enough, then, φu(s,0)<Jk(su+)+Jk(tu−)≤φu(s,t) for all s∈[0,ˉs]. Thus there is t0∈[0,ˉt] such that φu(s,0)≤φu(s,t0) for all s∈[0,ˉs]. Thus, (su,tu)∉[0,ˉs]×{0}. Similarly, (su,tu)∉{0}×[0,ˉt].
By direct computation, we get
for all (s,t)∈(0,ˉs]×(0,ˉt]. Hence, there holds
In view of (18), it follows that φu(s,ˉt)≤0∀s∈[0,ˉs] and φu(ˉs,t)≤0∀t∈[0,ˉt]. That is, (su,tu)∉{ˉs}×[0,ˉt] and (su,tu)∉[0,ˉs]×{ˉt}. Hence, we can deduce that (su,tu)∈(0,ˉs)×(0,ˉt). By (18), (19) and (20), we deduce
It is impossible. The proof of Case 1 is completed.
Case 2: B2=0. From the definition of Jk, it is easy to show that there exists t0∈[0,∞) such that φu(s,t)≤0, for all (s,t)∈[0,ˉs]×[t0,∞). Thus, there is (su,tu)∈[0,ˉs]×[0,∞) satisfying
We need to prove that (su,tu)∈(0,ˉs)×(0,∞). Similarly, it is noticed that φu(s,0)<φu(s,t) for s∈[0,ˉs] and 0<t≪1, that is (su,tu)∉[0,ˉs]×{0}. Also, for s small enough, we get φu(0,t)<φu(s,t) for t∈[0,∞), that is (su,tu)∉{0}×[0,∞). We note that
Thus also from (20) and B2=0, we have φu(ˉs,t)≤0 for all t∈[0,∞). Hence, (su,tu)∉{ˉs}×[0,∞). That is, (su,tu) is an inner maximizer of φu in [0,ˉs)×[0,∞). Hence by using (19), we obtain
which is a contradiction.
Since u±≠0, by Lemma 2.1, there are su,tu>0 such that ˜u:=suu++tuu−∈Mk. On the other hand, Fatou's Lemma follows that
By Lemma 2.1, we know 0<su,tu≤1. Since un∈Mk and B1=B2=0, we have
So, we have completed proof of Lemma 2.2.
3.
The proof of main results
In this section, we will prove main results.
3.1. The proof of Theorem 1.1
Proof. Since uk∈Mk and Jk(u+k+u−k)=ck, by Lemma 2.1, for (s,t)∈(R+×R+)∖(1,1), we have
If J′k(uk)≠0, then there exist δ>0 and θ>0 such that
We know by the result (15), if u∈Mk, there exists L>0 such that ‖u±‖>L and we can assume 6δ<L. Let Q:=(12,32)×(12,32), and g(s,t)=su+k+tu−k, (s,t)∈Q. In view of (21), it is easy to see that
Let ε:=min{(ck−¯ck)/4,θδ/8} and Sδ:=B(uk,δ), there exists a deformation η∈C([0,1]×E,E) satisfying
(a)] η(t,v)=v if t=0, or v∉(Jk)−1([ck−2ε,ck+2ε])∩S2δ;
(b)] η(1,(Jk)ck+ε∩Sδ)⊂(Jk)ck−ε;
(c)]Jk(η(1,v))≤Jk(v) for all v∈E;
(d)]Jk(η(⋅,v)) is non increasing for every v∈E.
To finish the proof of Theorem 1.1, one of the key points is to prove that
The other is to prove that η(1,g(Q))∩Mk≠∅. Let us begin this work. In fact, it follows from Lemma 2.1 that g(s,t)∈(Jk)ck+ε. On the other hand, from (a) and (d), we get
For (s,t)∈Q, when s≠1 or t≠1, according to (21) and (24),
If s=1 and t=1, that is, g(1,1)=uk, so that it holds g(1,1)∈Jck+εk∩Sδ, then by (b)
Thus (23) holds. Then, let φ(s,t):=η(1,g(s,t)) and
The claim holds if there exists (s0,t0)∈Q such that Υ(s0,t0)=(0,0). Since
and |s−1|2(6δ)2>4δ2⇔s<2/3ors>4/3, using (ⅰ) and the range of s, for s=12 and for every t∈[12,32] we have g(12,t)∉S2δ, so from (a), we have φ(12,t)=g(12,t). Thus
On the other hand, from (9) and u∈Mk, we have that
According to (f3), when 0<t<1, H(t,t)>0, while in the case t>1, H(t,t)<0. Similarly, it is easy to get G(t,t)>0fort∈(0,1),G(t,t)<0fort>1. By above discussions, due to (f3) and 2∗α>4, we have
which implies that
Analogously, φ(32,t)=g(32,t) implies that
that is,
By the same way,
From (25)-(27), the assumptions of Miranda's Theorem (see Lemma 2.4 in [17]) are satisfied. Thus, there exists (s0,t0)∈Q such taht Υ(s0,t0)=0, i.e. η(1,g(s0,t0))∈Mk. Comparing to (23), uk is a ground state nodal solution of problem (1).
3.2. The proof of Theorem 1.2
Proof. Recall that β=α3S32α, where S:=infu∈E∖{0}‖u‖2(∫R3|u|2∗αdx)22∗α. Similar to the proof of Lemma 2.2, we claim that there exists k⋆1>0 such that for all k≥k⋆1, there exists vk∈Nk such that Jk(vk)=c∗>0 and there is k⋆>0 such that c∗<β for all k≥k⋆. By standard processes, we can assume vn⇀vk∈E. Therefore, lim infn→∞Jk(tvn)≥c∗≥Jk(tvk)+t22A−t2∗α2∗αB+bt44(C2+2C‖vk‖2K), where A=limn→∞‖vn−vk‖2,B=limn→∞|vn−vk|2∗α2∗α and C=‖vn−vk‖2K.
Firstly, we prove that vk≠0. By contradiction, we suppose vk=0. The Case B=0 is contained in above equality. So we only consider the case B>0. The fact β≤α3(A1(B1)22∗α)32α follows that
Which is a contradiction.
Then, we prove that B=0 and c∗isachievedbyvk. Indirectly, we suppose that B>0. Firstly, we can maximize φvk(t)=Jk(tvk) in [0,∞) at tv, which be an inner maximizer. So we get t2v2A−t2∗αv2∗αB+bt4v4(C2+2C‖vk‖2K)>0. Thus from tvvk∈Nkb we get a contradiction by
From the above arguments we know ˜v:=tvvk∈Nk. Furthermore, we have
Therefore, tv=1, and c∗ is achieved by vk∈Nk.
By standard arguments, we can find vk∈E, a ground state solution of problem (1). For all k≥k⋆, the problem (1) also has a ground state nodal solution uk. Let k⋆⋆=max{k⋆,k⋆1}. Suppose that uk=u++u−, we let su+,tu−∈(0,1) such that
Thus, the above fact follows that













 DownLoad:
DownLoad: