1. Introduction
Mercury is a ubiquitous environmental toxicant that can cause many health defects for humans [1]. There are three distinct species of mercury: elemental, inorganic and organic with different uses and exposure routes, as well as causing different health effects [2]. Methylmercury exposure most commonly occurs from contaminated fish consumption [3]; inorganic mercury is primarily used in industrial settings and results in occupational exposures [4]; and metallic or elemental mercuryis commonly used in dental amalgams and small-scale artisanal gold mining.
Because mercury and mercuric compounds can cross the placenta and affect the developing fetus, these individuals are particularly at risk. Numerous studies in humans have examined the effects of higher level prenatal mercury exposure, with conflicting conclusions [5,6,7]. At least in mouse models, lower levels of mercury exposure can cause decreased performance in motor Function [8]. Moving beyond the neurodevelopmental effects, research focus is now shifting to the possible broader health complications that can arise from low-level prenatal mercury exposure, including effects on the immune system.
In our study we focus on mercury exposures, which are below the neurotoxic threshold and are closer to levels encountered by high fish-consumers (more than twice per week) in the United States [9,10]. While human exposures in the United States are primarily to methylmercury, we utilized inorganic mercury for a number of reasons. We have previously shown that both inorganic and organic mercury exposure is immunotoxic in vitro with similar patterns of response when human immune cells were exposed to mercuric chloride [11] or methylmercury [12]. Similarly, we have demonstrated that exposures to both inorganic (elemental) mercury [13] and methylmercury [14] can increase autoimmunity in humans. Finally, we utilized the same dosing protocol (both dose and route) with mercuric chloride in studies which demonstrated that inorganic mercury exposure increased the severity of autoimmune disease in susceptible mice [15,16]. Although these experiments were conducted using mercuric chloride, it should be noted that the chemical form of mercury found in fish is methylmercury cysteine, that is, the methylmercury bound to cysteine likely within a larger protein, which is less toxic than methylmercury chloride in studies on zebrafish [17].
Therefore, for these studies, we asked whether prenatal inorganic mercury exposure could cause programming changes in gene expression related to immune function. We hypothesize that these programming changes in the immune system might increase susceptibility to or severity of autoimmune disease at adulthood. In this study, we predicted that the gene expression changes that occur after prenatal exposure in these systems are programming changes that are long-lasting and will persist into adulthood with implications for adult health. To test this, we used BALB/c mice and exposed pregnant dams to inorganic mercury every other day from gestation day (GD) 5-15 and examined gene expression changes that occurred in the offspring.
2. Methods and Materials
2.1. Mice
BALB/c mice were purchased from Jackson Labs (Bar Harbor, ME). Animals were kept in specific pathogen-free conditions with standard light-dark cycles and fed a regular diet and water ad libitum. All procedures were approved by the Animal Care and Use Committee of the University of South Carolina.
2.2. Mercury exposure
Mice were established in breeding trios (one male, two females). Following the protocol of Silva et al. [18], female mice (n = 6 per treatment group) determined to be plug positive (GD0) were treated with HgCl2 in PBS by subcutaneous injection in the scruff of the neck from GD5 to 15, every other day, at a dose of 200 μg/kg bodyweight in a total volume of 100 μL. Thus, a 20 g dam received a dose of 4 μg HgCl2 every other day for 6 doses. We chose to use inorganic mercury (HgCl2) and the subcutaneous dosing route because this exposure is frequently found in the literature [19] although other studies have utilized HgCl2 in drinking water [20] in different strains of mice. As others have demonstrated differences in mercury toxicokinetics with mouse strains [21], and we routinely work with BALB/c mice and HgCl2 exposures, we deemed it important to keep these variables constant for comparisons within our own studies. This exposure protocol does not result in tissue damage at the site of injection (data not shown).
Control animals received an equal volume of PBS alone by the same route. Offspring from each dam were weaned at 4 weeks of age with no further mercury exposure. At 8 weeks of age, offspring (n = 6-8 per treatment group per sex) were euthanized by cervical dislocation under deep isoflurane anesthesia. Tissues, including spleen, were harvested for later analyses. Mercury exposure did not result in changes to the number of pups per litter (average was 6 across both treatment groups) or the sex ratio (both treatment groups had 50% male and 50% female offspring).
The entire experiment including prenatal exposure was replicated in two independent experiments. Since data were similar between replicates, we present results from one of the independent experiments. That is, the data presented here are from 6 dams per treatment group and 6-8 offspring per treatment group per sex.
2.3. Cytokine measurement
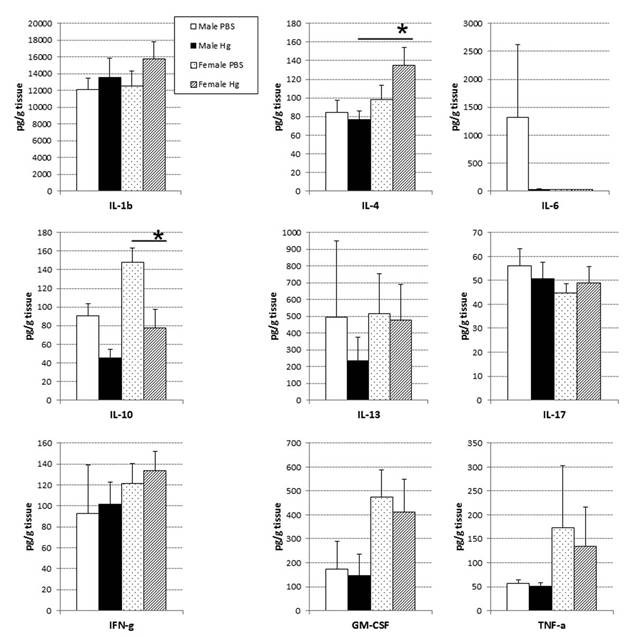
Half of the spleen was frozen in dry ice immediately after harvest and stored at− 80°C until processing. Tissues were weighed and homogenized in Minimal Essential Media (MEM) supplemented with 2% FBS (1% weight/volume) at 4 °C, and supernatants used for cytokine analysis. According to manufacturer’s instructions, cytokines were measured in tissue supernatants using bead-based multiplex cytokine enzyme-linked immunosorbent assays (Bio-Plex; Bio-Rad). The limits of detection for the panel of cytokines were as follows: interleukin (IL)-1β, 1.70 pg/mL; IL-4, 2.43 pg/mL; IL-6, 1.02 pg/mL; IL-10, 1.04 pg/mL; IL-13, 1.57 pg/mL; IL-17a, 1.44 pg/mL; interferon (IFN)-γ, 1.30 pg/mL; granulocyte-macrophage-colony stimulating factor (GM-CSF), 1.58 pg/mL; tumor necrosis factor (TNF)-α, 1.73 pg/mL. Cytokine protein levels are expressed as pg/g of tissue ±SEM.
2.4. RNA isolation and microarray analysis
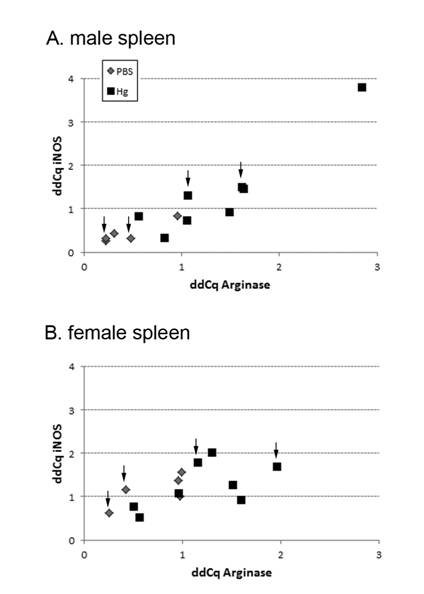
Spleens were used for microarray analysis. RNA and miRNA was isolated using miRNeasy Mini kit following the manufacturer’s protocol (Qiagen) and RNA purity and quantity was determined by 260/280 ratio (Synergy HT, Biotek). RNA integrity was assessed using Agilent 2100 bioanalyzer. Individual samples for each treatment group (n = 4 male, 4 female) were selected for high or low responder activity (n = 2 per sex) based on real-time PCR (qPCR) analysis of iNOS/arginase expression levels (Figure 1). These individual samples were then analyzed by microarray (Whole Mouse Genome Microarray 4× 44k v2, Agilent; SurePrint G3 Mouse miRNA8× 60k v17, Agilent). Gene and miRNA expression patterns were determined using Genespring GX software. For gene expression analysis, data were log(2) transformed, quantile normalized and the moderated T-test statistic used with a p-value of < 0.05 and fold change 1.5 as cutoffs. For miRNA analysis, data were log(2) transformed, 90th percentile shift normalized and the moderated T-test statistic were used with p-value < 0.05 and fold change 2.0 as cutoffs. Differentially expressed genes between treatment groups were utilized for subsequent qPCR validation and in silico analyses.
2.5. In silico analyses of gene expression patterns
Ingenuity Pathway Analysis (IPA; Ingenuity Systems, CA, USA) was used to generate IPA network data by inputting microarray data. Ingenuity generates networks by identifying published gene relationships and provides an IPA score (p value) indicating the likelihood that the significantly up or down-regulated genes found in our microarray would be present in a given network. We used these microarray data to inform examination of gene expression changes in the full cohort of male (n = 6-8 per treatment group) and female (n = 6-8 per treatment group) offspring from both treatment groups by qPCR.
2.6. Real-time PCR (qPCR)
Custom primers (Table 1) were designed for each target gene using Primer Blast Search (NCBI). RNA was reverse transcribed and amplified using a SensiFAST SYBR No-ROX One Step Kit according to the manufacturer’s protocol (Bioline). Gene expression patterns for each gene were normalized to expression levels of the housekeeping genes. Data are expressed as ΔΔCq based on the equation for normalized expression:
Table 1. qPCR primers
|
Gene
|
Forward Primer
|
Reverse Primer
|
|
iNOS
|
CCACCAACAATGGCAACATCAGGT
|
TAGGTCGATGCACAACTGGGTGAA
|
|
Arginase
|
TGGCTTTAACCTTGGCTTGCTTCG
|
AAAGAACAAGCCCTTGGGAGGAGA
|
|
Adiponectin
|
CCGGGACTCTACTACTTCTCTT
|
TTCCTGATACTGGTCGTAGGT
|
|
Vitronectin
|
GGACGTGTTCACTATGCCAG
|
TCCGTCCGAGGATTTAGGTC
|
|
TNFSF18
|
TCTGCTTTGAAACTGGCGTT
|
CCTTGGGGTAAAGTCGATGC
|
|
Complement Factor D
|
GTACCATGACGGGGTAGTCA
|
CACGTAACCACACCTTCGAC
|
|
IFN-γ
|
TGATTGCGGGGTTGTATCTG
|
GACATCTCCTCCCATCAGCA
|
|
RPLP0 (housekeeping)
|
CGTTGGAGTGACATCGTCTTTA
|
GGATGATCTTGAGGAAGTAGTTGG
|
Normalized Expression (sample gene of interest) =Relative Quantity of a Sample (Sample gene of Interest)/Relative Quantity of a Sample (reference gene)(1/n)
2.7. Statistical analysis
Statistically significant differences between treatment groups were analyzed using a two-tailed t-test. A value of p < 0.05 was considered significant. Modified Thompson tau test was used to check for outliers.
3. Results
3.1. Determining high and low expressors for subsequent microarray analysis based on arginase and iNOS expression
The goal for our microarray analysis was to not pool samples, but use a small subset of individuals to establish gene expression changes induced by prenatal mercury exposure. In order to select individuals for microarray analysis, we used qPCR for two genes related to macrophage function, inducible nitric oxide synthase (iNos) and arginase (Figure 1). The lowest expressors in the PBS treatment group and highest expressors in the mercury treatment group were selected for each sex and the RNA from the spleens of these individuals was used in the subsequent microarray analysis (n = 2 per treatment group per sex, denoted with arrows in Figure 1). We chose not to use the highest expressor from the males (upper right corner of Figure 1A) for the microarray analysis as it was found to be an outlier based on the modified Thompson tau test; however, this sample was included in other analyses presented (cytokines, etc) since it was not an outlier in those analyses.
3.2. Mercury alters gene expression differently in males and females
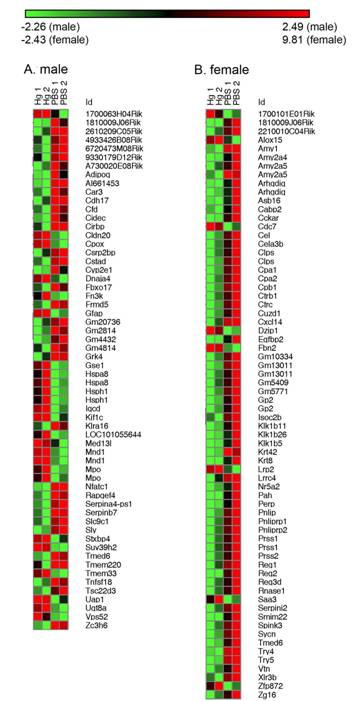
From the total RNA isolated, mRNA expression changes were examined by microarray analysis. Prenatal mercury exposure induced a number of changes to mRNA (Figure 2) expression levels and these changes varied between males and females. In males, out of 24,562 known genes on the array, 446 genes were up or down-regulated at least 1.5-fold with prenatal mercury exposure. However, when examining the number of genes where expression was changed at least two-fold, this number is reduced to 60. In females, the total number of genes changed was much smaller than the males with a total of 138 genes up or down-regulated at least 1.5-fold, with 68 of these changed at least two-fold. There was very little similarity between the genes modulated in males and females, as there were only 6 genes in common modulated more than 1.5-fold and only 2 in common modulated more than two-fold. Interestingly, the gene expression array (Figure 2) did not show a difference in iNOS for either males or females as we would have predicted based on the qPCR analysis (Figure 1).
3.3. IPA analysis reveals a number of immunological changes caused by prenatal mercury exposure
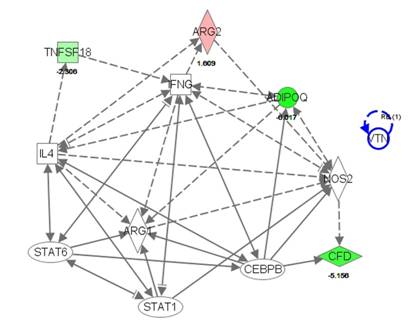
The data from the microarray were visualized in silico using Ingenuity Pathway Analysis. With this program, we examined the changes in gene expression using the total dataset from both males and females. While a number of changes were present in gene expression, because of limited similarities between males and females we chose to create a pathway based on the expressor genes arginase and iNOS (Figure 3). From there, we were able to create a pathway that included 12 genes of interest, 11 of which are involved in apoptosis, 9 involved in fibrosis and cell movement of leukocytes, 8 involved in macrophage activation and 5 in bacterial infection (Table 2). These are the genes that we chose to focus on in qPCR validation of the total male and female cohorts (n = 6-8 per treatment group per sex). As noted, the gene expression arrays did not find differences in the subset of samples tested for iNOS, though we would have predicted differences based on the qPCR data (Figure1) for this gene. We included this gene in our pathway, but it is uncolored to denote no difference between treatment groups.
Table 2. IPA pathway genes of interest and related biological processes
| Abbreviations: AdipoQ: adiponectin, Arg1: arginase 1, Arg2: arginase 2, CEBPB:CCAAT/enhancer binding protein beta, CFD: complement factor D, IFNg: interferon-γ, IL4: interleukin-4, Nos2: nitric oxide synthase 2, VTN: vitronectin |
|
|
Apoptosis
|
Fibrosis
|
Cell Movement of Leukocytes
|
Macrophage Activation
|
Bacterial Infection
|
|
AdipoQ
|
|
|
|
|
|
|
Arg1
|
|
|
|
|
|
|
Arg2
|
|
|
|
|
|
|
CEBPB
|
|
|
|
|
|
|
CFD
|
|
|
|
|
|
|
IFNg
|
|
|
|
|
|
|
IL4
|
|
|
|
|
|
|
Nos2
|
|
|
|
|
|
|
STAT1
|
|
|
|
|
|
|
STAT6
|
|
|
|
|
|
|
TNFSF18
|
|
|
|
|
|
|
VTN
|
|
|
|
|
|
3.4. Males are more sensitive to gene expression changes with mercury exposure than females
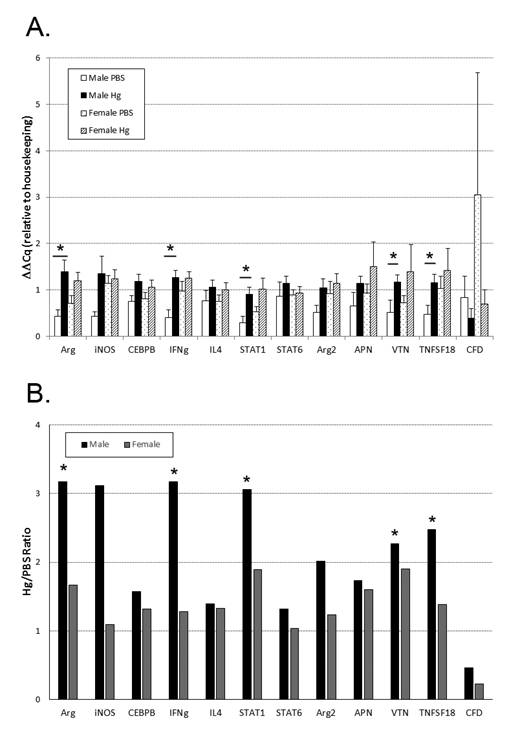
From the IPA pathway generated, we chose to validate gene expression changes specifically related to the immune system (Table 2). Using qPCR, we found a significant increase in the expression of arginase, vitronectin, TNFSF18, Stat1, and IFN-γ in the mercury exposed males compared to PBS exposed males (Figure 4A). There were no significant changes in the expression of these same genes or others examined in females (Figure 4A). Differentially expressed to focus on comparing gene expression changes between males and females, we calculated a ratio of the average gene expression level in mercury-treated divided by control-treated from each sex. We saw a greater change in males in all genes examined compared to females (Figure 4B).
3.5. Mercury exposure differentially alters epigenetic (miRNA) regulator levels in males and females
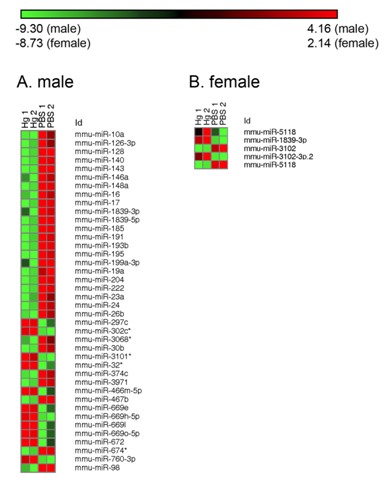
For the miRNA array a similar trend was observed in that there were no similar miRNAs altered between males and females. Out of 1136 total miRNAs on the array, 43 miRNAs were up or down-regulated at least two-fold with prenatal mercury exposure (Figure 5) in males. As for mRNA, in females the total number of miRNAs changed was much smaller than the males. A total of 5 miRNAs were up- or down-regulated at least two-fold in the females, and none were the same as those changed in males. From those genes selected for qPCR, 3 are affected by 2 different miRNAs. In males, miR-191-5p had a decreased fold change (25.8) and can affect CCAAT/enhancer binding protein beta (CEBPB). In females, miR-1188-3p was increased (3.6) and has two targets—iNOS and adiponectin.
3.6. Prenatal mercury exposure induced little change in baseline cytokine protein levels
In an attempt to link changes in gene expression changes to protein levels, we performed a cytokine analysis using spleen homogenates. Interestingly, there were no observed differences in terms of splenic cytokine protein levels in males, but females showed a significant decrease in IL-10 and increase in IL-4 with prenatal mercury exposure (Figure 6).
4. Discussion
Previous studies have focused on the effects of mercury exposure on the nervous system, and in particular, the effects of prenatal exposures on the developing fetus. Prenatal mercury exposure not only causes neurological disorders, but cardiovascular disease and immune modulation [22,23,24]. Most rodent studies have been conducted using doses of mercury that would be considered neurotoxic in humans and at exposures that are generally not achievable in humans through common routes of exposure such as contaminated fish consumption [25,26]. Our study used lower level inorganic mercury exposure, comparable to the upper end of normal methylmercury exposures in the United States [9]. We focused on whether this exposure induced lasting changes in gene expression in the offspring. We predicted that changes in gene expression alter adult response to triggers of immune disorders and might increase severity or susceptibility to autoimmune disease. We have previously demonstrated that adult exposure to these same levels of inorganic mercury alters the disease progression of coxsackievirus-induced autoimmune myocarditis, increasing severity of the disease in both males and females [15,16] and increasing the prevalence of dilated cardiomyopathy in males [15]. If prenatal exposure to mercury results in changes in gene expression, then those individuals who subsequently manifest autoimmune diseases might be at higher risk for exacerbated disease conditions. Our data suggest that there are differences between males and females such that females are protected from the effects of prenatal mercury exposure on the immune system. It must be noted that we utilized a biased selection process in order to choose the samples for microarray analysis. While we validated those genes identified as highly different between the groups with qPCR using all the offspring from the experiments, the biased selection and subsequent microarray analysis could have generated a skewed picture of gene expression differences. Thus, the conclusions presented must
be taken in this light.
We saw many genes that had significant altered expression in the microarray (at least two-fold change compared to baseline) and this number was similar between males and females. However, for most of the genes we examined the overall change with mercury exposure was higher in the males compared to the females. One characteristic that these genes shared was their role in inflammation or being more pro-inflammatory in nature, suggesting that prenatal exposures to mercury could predispose males to exacerbation of response to autoimmune triggers later in life.
Two well-known pro-inflammatory mediators are IFN-γ and STAT1. Both are important in the stimulation of macrophages toward an M1, or pro-inflammatory, subtype [27]. These macrophages produce inflammatory cytokines including IL-1β, TNF-α, and IL-6 [28]. While our cytokine data did not demonstrate differences in these cytokines in the spleen, we did not separate out cell subtypes which may account for some of this discrepancy. Since males had a greater increase in these two genes (IFN-γ and STAT1) with mercury exposure, this gene expression pattern may indicate that males exposed to mercury had a higher percentage of M1 macrophages present compared to controls and to females.
Another gene that has a role in inflammation and nitric oxide production is TNFSF18. This glucocorticoid induced ligand is expressed on numerous antigen presenting cells including macrophages, dendritic cells and B-cells [29]. Expression of TNFSF18 is mediated by NF1 [30] and expression can be increased by pro-inflammatory stimuli and T-cell activation [31].
Vitronectin is a glycoprotein involved in multiple biological functions, including complement interactions, coagulation and the innate immune response. It is detected in most cells and tissues and is typically a sign of inflammation or stress [32]. Demonstrated by Seiffert et al. [33], vitronectin is up-regulated by IL-6 in both acute and chronic inflammation, and in vitro exposure to mercury has been shown to increase IL-6—although this was not demonstrated in our model [34]. Along with fibronectin, vitronectin has a role in bacterial colonization, infection, and then phagocytosis [35]. In the complement pathway, it is a known membrane attack complex inhibitor believed to act to protect against unnecessary cell lysis [35]. In the males exposed to mercury prenatally, we saw a larger increase in vitronectin expression compared to females—again hinting that the males have a higher level of inflammation present in the spleen compared to the females.
The only miRNA altered that has a role in our genes of interest is miR-191-5p, which was decreased in expression 25 fold in males. This miRNA interacts with CEBPB, which is one of the few genes we validated that did not see a large change between mercury and control treatments in males or females. However, miR-191-5p is one of three miRNAs that have been demonstrated to be differentially expressed in the T regulatory cells of patients with Type 1 Diabetes [36]. This could implicate another scenario for increased inflammation in males compared to females.
Through the analysis of cytokine production, we saw a number of cytokines that were changed, but IL-10 and IL-4 were the only cytokines significantly affected. IL-10, along with IL-4, are anti-inflammatory cytokines that inhibit Th1 T cell responses [37,38]. Reduction in IL-10 in mercury-treated females could be an indicator of increased inflammation, and a similar response was demonstrated by Silva et al. [18], in that splenocytes of adult females prenatally exposed to mercury produced less IL-10 upon in vitro stimulation. However, where Silva et al. witnessed a decrease in the production of IL-4 by thymocytes from prenatally exposed mercury-treated females, we saw a significant increase in IL-4 levels in the spleen. These differences might be due to organ-specific effects of prenatal mercury exposure or to the differences in experimental design; that is, differences in levels of cytokine measured in the tissue versus cytokines produced by stimulated cells in vitro.
5. Conclusion
Overall, when comparing the gene expression changes caused by prenatal mercury exposure between males and females, it appears to be that males have a much stronger push toward inflammation to which females are not as susceptible. Previously demonstrated by Silva et al., prenatal exposure to mercury has numerous effects on the immune system that are sex dependent [18]. While there are still a number of genes that need to be validated including the pro-inflammatory cytokine IL-6, we present evidence demonstrating that the effects of prenatal inorganic mercury exposure can affect the fetus differentially depending on sex. Future studies will utilize oral exposures to methylmercury to more closely model human exposures from contaminated foods such as rice and predatory fish.
Acknowledgements
This work was funded by the National Institutes of Health [K99/R00 ES015426 to J.F.N.].
Conflict of interest
All authors declare no conflicts of interest in this manuscript.









 DownLoad:
DownLoad: