Abbreviation: NPIs: non-pharmaceutical interventions; IFR: infection fatality risk
1.
Introduction
The COVID-19 pandemic has had a profound impact on social life and life expectancy worldwide. Since its emergence in late 2019, COVID-19 has caused 6.8 million deaths, as of 1 February 2023 [1]. Various countermeasures have been taken to curb the epidemic. Countermeasures are classified into pharmaceutical interventions including vaccination and antiviral treatment, and non-pharmaceutical interventions (NPIs) including social distancing, using personal protective equipment (e.g., masks), quarantine, and case isolation. In most countries and regions, all possible options have been used to stop the spread of the disease [2,3]. Stringent NPIs were put into place worldwide, and primary series vaccination was completed within 2021, or at least by mid-2022, in many countries. However, in late 2021, the SARS-CoV-2 Omicron variant (B.1.1.529) emerged and spread rapidly around the globe [4]. Although the mortality impact of the Omicron variant was minimized mainly owing to vaccination and treatment [5], the variant led to substantial morbidity and mortality around the world.
Since the initial interventions in February 2020, China adopted a containment strategy, the "zero-COVID policy", in response to the pandemic [6]. Despite its early success, the policy has involved mandatory lockdowns, i.e., restricting people to their homes or within a limited geographic space. Such interventions led to debates over adverse impacts (e.g., harm to mental health and reports of domestic violence [7,8]) and human rights and freedom. Moreover, the sustainability of the zero-COVID policy was questioned owing to its economic cost [9], requirement for RT-PCR testing resources, and deterioration of trade and business opportunities (e.g., blockades in Shanghai [10]). After the lockdown policy was maintained for more than 2.5 years, widespread protests have occurred [11], and it became unrealistic for the Chinese government to continue strict NPIs as of late 2022.
As part of the implemented pharmaceutical interventions, a mass vaccination program with its originally manufactured inactivated vaccine, adenovirus vector vaccine, and recombinant protein vaccine has been implemented in China [12]. An official statement from the National Health Commission indicated that the first dose for people over 60 years old attained a coverage of approximately 90% by November 30, 2022, but the coverage of additional doses (i.e., second and third doses) was not substantially elevated among elderly people by late 2022, especially those aged 80 years [12].
Since the beginning of the pandemic, the mortality impact of COVID-19 in China has been studied by numerous researchers [13,14,15,16]. In particular, given the substantial growth rate of the Omicron variant and its repeated importations into China [17], the possible mortality impact upon reopening of Chinese society has become important to consider. According to a published study on the Omicron variant by Cai et al. [16], the cumulative number of deaths in China could be on the order of 1.55 million deaths according to an age-structured stochastic compartment model [16]. As the mortality impact could be substantial, several research studies attempted to estimate the excess mortality, using existing methods including time series and machine learning techniques [18,19]. However, such investigation usually requires use of a sophisticated model to capture the detailed transmission dynamics. A simple and convenient approach to address this issue has been the subject of ongoing research. Moreover, the question has arisen regarding whether the mortality impact could be partly eased by increasing the COVID-19 vaccination coverage or by using mRNA vaccines instead of inactivated ones.
In the meantime, epidemiological transmission models have been extensively studied with the aim of estimating the cumulative incidence of infection, including the instances where vaccination takes place [20]. The cumulative risk of infection was shown to be derived from by the so-called final size equation, an explicit, but non-closed form solution of the transmission model with respect to the fraction of people experiencing infection [21]. To date, the final size equation provides a comprehensive and reasonable pathway to predict the cumulative incidence of an epidemic in the absence of time-dependent countermeasures, and the equation has been employed for various applications [22,23,24]. Estimating the mortality impact can help improve our understanding of the magnitude of the COVID-19 pandemic, thereby allowing policymakers to make value judgments. The purpose of the present study was to formulate an age-dependent final size equation to easily allow computation of the cumulative risks of infection and death in China under various scenarios of vaccination and the use of different types of vaccine. In this study, we applied a next-generation matrix to capture the age-dependent heterogeneous transmission of COVID-19 infection.
2.
Materials and methods
2.1. Vaccination coverage, census, and infection fatality risk (IFR)
As of 14 December 2022, the National Health Commission announced that 3.4 billion vaccine doses had been administered in China [25]. The coverage of the second dose in the age groups 0–59, 60–79, and 80 years and older has been documented as 86.0%, 89.8%, and 66.4%, respectively [25]. The coverage of the third dose in these age groups is reported to be 52.3%, 72.3%, and 42.3%, respectively [25]. The first dose, mainly administered from December 2020 [26], is expected to have had a very limited impact on modifying the transmission dynamics of the Omicron variant in late 2022; thus, this dataset was discarded. Age was dealt with as a discrete group, and the population was divided into 10-year age groups (those aged 80 years and older were grouped together as the single oldest category). The age-specific population size was retrieved from World Population Prospects 2022 published by the United Nations (Supplementary Table 1) [27].
To compute the expected number of deaths among infected individuals, we used the age-specific infection fatality risk (IFR) extracted from O'Driscoll et al. (Supplementary Table 2) [28]. The IFR was defined as the fraction of deaths among all infected individuals. As for the age-dependent transmission dynamics, we used an age-dependent next-generation matrix in Japan (Supplementary Table 3) [29]. Table 1 shows the parameter values and references used. For simplicity, we assumed that the effectiveness against infection and severe disease (or death) of the second and third doses of inactivated vaccine is independent of age. We assumed that the effectiveness against infection conferred by the second dose could take the value of 0%, 10%, or 20%, referring to the fact that the risk of infection after receiving second-dose vaccines did not deviate substantially from the risk with no vaccination [30]. As the baseline assumption, the basic reproduction number (R0) of the Omicron variant was assumed to be 3.4, referring to a published simulation study [9]; however, we varied R0 broadly from 1 to 10. When examining a scenario in which mRNA vaccines are used, we assumed that mRNA vaccine BNT162b2 replaced everything with identical coverage, as mentioned above.
2.2. Final size equation with vaccination
Here, we used the "final size equation" that can usually be derived from a structured transmission model (e.g., susceptible–infectious–recovered [SIR] model) [37]. That is, we used the recursive equation that allowed us to iteratively calculate the cumulative number of infections throughout the course of the epidemic. Here, the final size is defined as the fraction of population that experienced infection by the end of an epidemic. The age-dependent final size equation is derived from an age-structured SIR model; the derivation process can be found elsewhere [38]. Letting zi be the final size of age group i,
where Rij is the element of the next-generation matrix representing the average number of secondary infections in age group i produced by a single infected individual in age group j.
Usually, if there are two or more different types of host, as defined by vaccination doses (i.e., unvaccinated, one-dose, two-dose, and three-dose), using a vector of infected individuals would be called for; thus, the elements of the next-generation matrix would be increased. However, we simplified the modeling approach by imposing an assumption that vaccination mainly reduces susceptibility only and also that contact behavior of the host is independent of vaccination doses. We supposed that the two-dose and three-dose population size is ps, a and pk, a, respectively, and the remaining groups (unvaccinated and one-dose) are grouped together with a fraction 1- ps, a-pk, a. We supposed that the unvaccinated and one-dose group had a hazard of infection λ(t) at calendar time t, the two-dose group had a reduced hazard (1−vs,inf)λ(t), and the third-dose group had the hazard (1−vk,inf)λ(t). Because the susceptible fraction at the beginning of the epidemic is proportional to the above-mentioned coverage-dependent population size, the total incidence is described as:
That is, under the above-mentioned assumption, the hazard rate (or equivalently in this case, the transmission rate) of the entire population is reduced by a factor of 1−(ps,avs,inf+pk,avk,inf).
Using the simplification in equation (2), we computed the age-dependent final size, including the vaccination effectiveness, as:
We estimated the cumulative incidence of infection (zi) from equation (3).
The cumulative risk of death can be then obtained by multiplying the IFR fi to the cumulative incidence zi. Let vs,death and vk,death represent the vaccine effectiveness against fatal infection among those who completed second and third dose immunization, respectively. Accounting for the vaccine effectiveness in escaping from fatal infection, and assuming that the protection from severe infection was conditionally independent from the risk of infection (as reflected in zi), the cumulative number of deaths in age group i (Di) by the end of the endemic was calculated as:
where fi is the IFR of age group i and Ni is the population size of age group i. The final size of age group i by vaccine history, i.e., z0, i, zs, i and zk, i, were obtained as the solution of following equations:
which can be derived in the same way of equation (3).
In this study, we sought to explore the impact of different values of R0, different vaccine coverage rates, and different types of vaccine on the cumulative risk of COVID-19 infection. To do so, we first calculated the mortality in each age group, varying the R0 from 1 to 10. To vary the R0, the next-generation matrix was normalized, and then R0 was multiplied to all elements. Next, we computed the mortality in the event that the third-dose coverage was 20% lower or 20% higher than the actual value for all age groups. The number of deaths that would be averted by enhanced implementation of the third-dose program was calculated. Third, we calculated the mortality in the event that an mRNA vaccine (BNT162b2) was used instead of inactivated vaccines, varying the R0 from 1 to 10. The mortality impact was compared between mRNA and inactivated vaccines.
2.3. Ethical considerations
This study used only publicly reported data, and we did not handle any personally identifiable information. Accordingly, the present study did not require ethical approval.
3.
Results
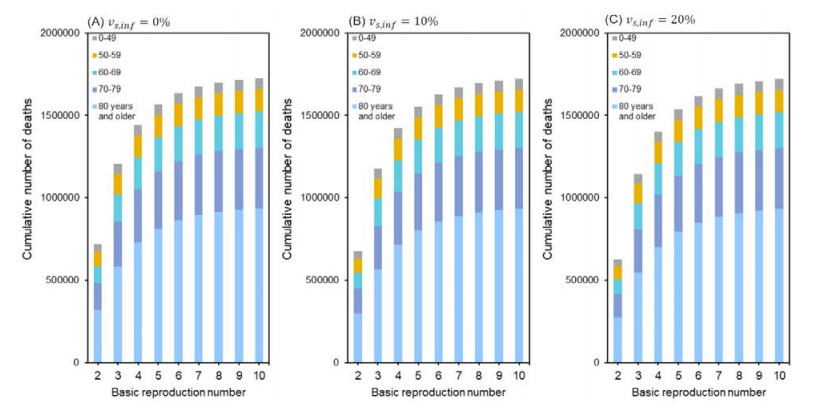
Figure 1 shows the cumulative number of deaths with varying the R0 from 1 to 10 for an assumed second-dose effectiveness at 0%, 10%, and 20%, respectively. For these values, with the default value of R0 at 3.4, the cumulative number of deaths was approximately 1.47, 1.46, and 1.44 million deaths, respectively. People aged 80 years and older accounted for approximately half of the expected deaths, and people aged 70–79 years accounted for 22%. As the R0 was increased, especially from 2 to 6, the expected number of deaths became substantially elevated.
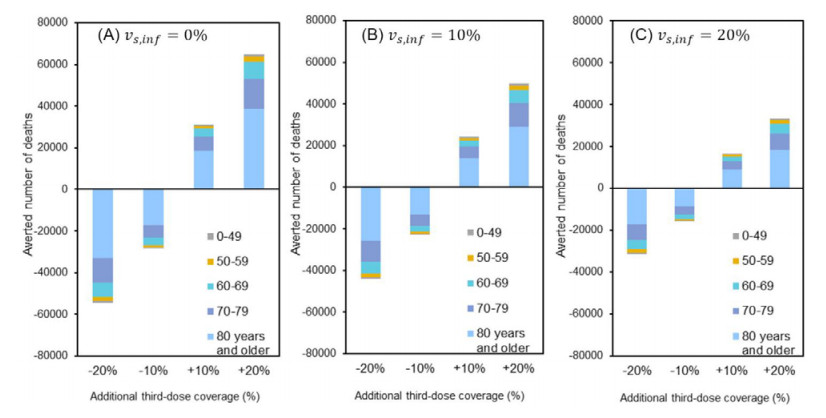
Figure 2 shows the number of deaths that would be averted by additionally increasing the coverage of third-dose vaccination. A 10% random increase in third-dose coverage prevented 30,948, 24,106, and 16,367 deaths with second-dose effectiveness of 0%, 10%, and 20%, respectively. Similarly, for these effectiveness values, a 20% random elevation in the third-dose coverage would save 64,868, 49,717, and 33,228 lives, respectively. Conversely, a 10% decrease in third-dose coverage led to an increase of 28,337, 22,732, and 15,899 deaths, respectively.
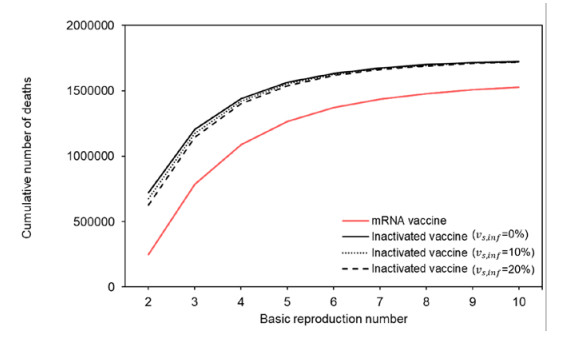
Figure 3 shows the cumulative number of deaths if mRNA vaccine (BNT162b2) were used instead of inactivated vaccines. Unlike the 1.4 million deaths using inactivated vaccines, the cumulative number of deaths was calculated to be 1.1 million deaths with an R0 of 3.4. Similar to inactivated vaccines, people aged 80 years and older accounted for approximately half of all deaths and those aged 70–79 years accounted for 20% of all deaths.
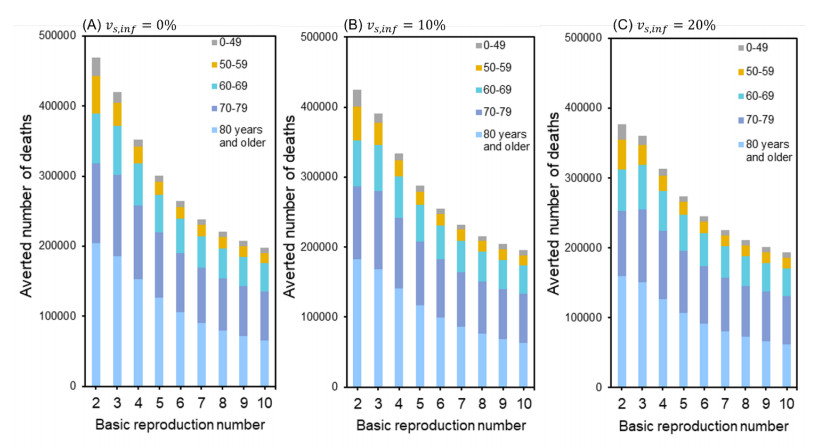
Figure 4 shows the number of deaths that could be averted if inactivated vaccines were replaced with mRNA vaccines. With mRNA vaccines and an R0 of 3.4, the number of lives that would have been saved was 343,000, assuming that second-dose effectiveness was 0%. Similarly, for second-dose effectiveness of 10% and 20%, the averted number of deaths would be 325,000 and 306,000, respectively.
4.
Discussion
In China, the COVID-19 containment strategy was changed in December 2022 [39], although a substantial number of elderly people remained unvaccinated. A similar epidemic trend of the 2022 BA.2 wave previously occurred in Hong Kong, with 71.1% of deaths occurring among people aged 80 years and older [40]. With the final size equation, we used a simple approach to assess the potential disease burden in China. We also explored how the massive number of deaths could be reduced by increasing the third-dose vaccination coverage before lifting restrictions and also by replacing inactivated vaccines with mRNA vaccine. Using the default value of R0 at 3.4, the cumulative number of deaths was calculated to be 1.4 million. We found that a 20% increase in third-dose coverage could prevent 33,000 to 65,000 deaths. If mRNA vaccine were used, approximately 300,000 lives could have been saved.
To the best of our knowledge, the present study is the first to have shown that the final size equation can conveniently be used to aid risk assessment. Although the approach was simplistic in calculating the mortality impact, the value of approximately 1.4 million deaths was consistent with published estimates in advance of reopening [16], and also with findings of a real time study in Beijing [41]. Cai et al. [16] estimated the total number of deaths in China using the age-structured stochastic compartment model which is governed by the age-dependent next-generation matrix. Leung et al. [41] estimated the fraction of the population infected from 1 November 2022, using a dynamic transmission model with real-time mobility data. Other studies included the one estimating the mortality impact using travel patterns during the course of epidemic [42] and the other one that tracked diagnosed case data from December 2022 to January 2023 [43]. Not only calculating the expected number of deaths in the default setting but also using the equation allowed us to implement risk assessment with additional or varied interventions (e.g., with additional vaccination). Apart from its simplicity, an important disadvantage of the final size equation is that fixed parameter values must be determined at the beginning of an epidemic. Even additional interventions must be assumed to be completed in advance of a major epidemic. Thus, if a drastic change that occurs during the course of an epidemic is to be studied, more sophisticated structured models [16,41] are required.
By examining possible interventions before lifting restrictions, our study showed that the number of deaths in China could be substantially reduced by increasing third-dose vaccination. The Chinese government issued a circular aimed at increasing the third-dose coverage among elderly people in December 2022 [12]. The present study findings endorse the scientific appropriateness of that real-time effort. By the end of January 2023, according to official reports, 92% of elderly people had completed booster vaccination against a population receiving second doses (inactivated vaccine) or first doses (adenovirus vector vaccine) of vaccine [44]; the third-dose coverage remains unknown. According to our results, even with very low effectiveness of the second dose, vaccination with the third dose would substantially contribute to protecting vulnerable people aged 80 years and older.
We also compared the mortality impact between mRNA and inactivated vaccines. Using an mRNA vaccine could have reduced the cumulative risk of infection by approximately 25%. The lesson to be learned from this simple exercise is that governmental decisions on the type of vaccine used in nationwide vaccination programs has a profound population impact, especially in the case of vaccine effectiveness being greatly reduced by antigenic evolution of circulating variants. The mRNA BNT162b2 vaccine as well as other mRNA vaccines (AZD1222) would substantially avert the risk of death owing to COVID-19 [32]. Some studies have shown that heterologous booster vaccines are more effective against infection and death than homologous booster vaccines [33,45,46]. Not necessarily having all three doses but having a single booster of mRNA vaccine might substantially lower the disease burden.
This study had five technical limitations. First, we assumed that the age-specific IFR remained constant throughout the course of the epidemic. Owing to a massive number of cases caused by circulating variants [47,48], health care facilities might have been overwhelmed with severe cases, thereby elevating the IFR [49]. The IFR may also decrease during the course of an epidemic due to widespread supply of antivirals and vaccines, and in that sense, an assumption of employing constant IFR could also have led to overestimation of the cumulative number of deaths. Second, our study did not precisely account for the time that has elapsed since the latest round of vaccination. In this sense, consistently using the assumed effectiveness values from the literature throughout the present study could have led to overestimating the preventive effect. Third, a fourth dose of vaccination remains very rare in China and was therefore not considered. If the booster program was actually widespread and effective, our study could have overestimated the final size. In fact, although not widely disseminated to the entire population, the Chinese government announced a fourth dose vaccination program starting on December 14, 2022 [50]. Fourth, the next-generation matrix used in this study was derived from our empirical study in Japan. This requires us to impose an important assumption that age-dependent contact patterns are comparable between Japan and China, which must be validated in the future. If infection control measures have resulted in less frequent overall contacts in China, our assumption may have led to an overestimation of the final size. Fifth, we did not explicitly account for antiviral treatment, again potentially overestimating the cumulative number of deaths. By January 5, 2023, the Chinese government had released the 10th edition of the treatment protocol for SARS-CoV-2 infection, which recommends the use of Paxlovid, Azvudine, and molnupiravir [51]. However, health care services were overwhelmed, and thus this expensive treatment was not sufficiently disseminated to high-risk patients in the early stage of illness; antiviral treatment may have reduced a part of the calculated risk of death.
More practical models should account for geographic heterogeneities. Lastly, given that contact behaviors and mobility are likely to have dramatically changed during the course of the epidemic, precise estimates require us to employ a dynamic model that accounts for time-dependent human mobility and case importations from other geographic units.
5.
Conclusions
Using the final size equation approach, we calculated the possible population impact of COVID-19 in China after reopening to be 1.4 million deaths. The increase in third-dose vaccination coverage contributed to decreasing the cumulative number of deaths by 30,000–60,000. If mRNA vaccines were used, the cumulative number of deaths could have been 1.1 million.
Acknowledgments
H. N. received funding from Health and Labour Sciences Research Grants (20CA2024, 20HA2007, 21HB1002, and 21HA2016), the Japan Agency for Medical Research and Development (JP 23fk0108685), JSPS KAKENHI (21H03198 and 22K19670), the Environment Research and Technology Development Fund (JPMEERF20S11804) of the Environmental Restoration and Conservation Agency of Japan, Kao Health Science Research, the Daikin GAP Fund of Kyoto University, the Japan Science and Technology Agency SICORP program (JPMJSC20U3 and JPMJSC2105), and the RISTEX program for Science, Technology, and Innovation Policy (JPMJRS22B4). We thank Analisa Avila, MPH, ELS, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Conflict of interest
The authors declare that they have no competing interests.









 DownLoad:
DownLoad: