1. Introduction
Much attention has been focused on the use of glycerol as a solitary carbon sauce in recent years for bio-based, industrial chemical production. This is because glycerol is an abundant, inexpensive carbon source, with a high degree of reducing potential and it is currently being generated as a byproduct of biofuel industry [1]. Glycerol could be converted into a value added succinic acid by using metabolically engineered Escherichia coli strains. Succinic acid is a potential platform chemical that could serve as a precursor for the synthesis of other commodity and specialty chemicals with a wide spectrum of applications [2,3].
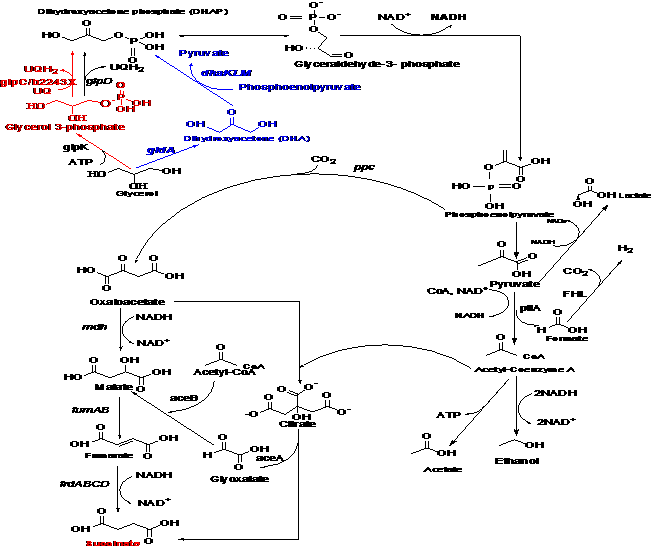
Metabolic engineering of Escherichia coli for increased succinate production from glycerol has progressed significantly using an experimental trial by error approach [1,4,5,6,7,8,9]. One of the notable examples was reported by Gonzalez and colleagues [1], where they introduced a heterologous pyruvate carboxylase (pyc) from Lactococcus lactis into E. coli for the production of succinate using glycerol as the substrate in minimal salt medium under micro-aerobic conditions. The succinate synthesis was achieved by the blockage of competing metabolic byproducts [1]. In E. coli central metabolism, glycerol utilization for succinate production proceeds via three (3) different routes to dihydroxyacetone phosphate (DHAP) which are: (1) the aerobic GlpK-GlpD respiratory route, (2) the anaerobic GlpK-GlpABC respiratory route (Figure 1) and (3) the GldA-DhaKLM fermentative route (Figure 1) [1,6]. We leveraged the knowledge base created in the aforementioned studies to investigate the underlying metabolic role of glpC via in silico gene knockout, in relation to E. coli succinate production and NADH regeneration under anaerobic conditions when glycerol is the only available substrate.
The advances seen in systems metabolic engineering and high throughput technology led to the accumulation of a wealth of biochemical data; where biochemical reaction networks were assembled into genome scale metabolic models (GEM) [9,10,11]. Genome scale metabolic reconstructions of E. coli have been developed [12,13] and used widely for predicting growth phenotypes in various environmental and/or genetic perturbations [14,15,16,17]. Model-driven metabolic engineering strategies can be performed using a variety of substrates and different computational tools such as OptFlux [18] and COBRA [19] to mention but a few. A more recent study reports a workflow methodology that uses model-driven biological discovery to understand certain underlying metabolic functions of some selected genes in E. coli [20].
A number of research efforts involving engineering of E. coli host metabolic pathways and succinic acid overproducing Mannheimia succiniciproducens have been conducted using genome comparison and in silico metabolic gene knockout with MetaFluxNet to increase succinate production in chassis E. coli [21]. In a different study [22], elementary mode analysis for the rational design of efficient glycerol conversion to succinate by E. coli was conducted using METATOOL 5.1 [23]. The approach investigated the pathway involved for optimal succinate production coupled with the effects of oxygen levels in relation to succinate production in E. coli [22]. Taken together, many investigators have applied experimental trial and error genetic engineering approaches to improve succinate production in E. coli using different strategies; ranging from addition of foreign genes [24,25,26,27,28] to metabolic evolution of energy conserving pathways using glucose as the solitary carbon source [29]. Other researchers [30] have constructed several strains of E. coli, in which genes involved in succinate competing pathways were knocked out without introduction of foreign genes. This strategy combined with several rounds of metabolic evolution, led to E. coli strains, designated as KJ060 (ΔldhA, ΔadhE, ΔackA, ΔfocA, ΔpflB and KJ073 (ΔldhA, ΔadhE, ΔackA, ΔfocA, ΔpflB, ΔmgsA ΔpoxB) with improved succinate production from a glucose substrate. As promising as this approach might be, there are, as yet, no E. coli strains or models lacking the glpC gene which have been reported to have increased succinate production using glycerol as the solitary carbon source. Here, we report a model-driven in silico glpC/b2243 metabolic gene knockout strategy for increased succinate production using an accurate genome scale metabolic reconstruction of E. coli iJO1366 [13] with the OptFlux software platform [18]. Minimization of Metabolic Adjustment (MOMA) was used as the gene knockout simulation algorithm [31] under the OptFlux software interface. The results obtained herein demonstrate, for the first time, that the mutant model lacking the glpC gene is predicted to increase succinate flux , when glycerol is used as the substrate, by about 30% (see Table1) when compared to the wild-type control model, . It was thus hypothesized that an additional molecule of NADH was generated following the glpC gene knockout and/or the glycerol central metabolism was channeled via the preferred GldA-DhaKLM fermentative route for increased generation of DHAP, PEP and subsequently, succinate (Figure 1).
2. Materials and Method
2.1. Model and flux balance analysis
The E. coli genome scale metabolic model developed by Orth and colleagues [13], designated as E. coli iJO1366 was utilized as a basis for metabolic gene knockout simulation, succinate yield, and strain design. This model was shown to be capable of accurate predictions of growth rates, metabolite excretion rates on a variety of substrates, and genetic conditions consistent with experimental data [12,13,15]. For all phenotype simulations, flux balance analysis was conducted. All computations were carried out using the OptFlux software platform version 3.07 (http://www.optflux.org), which was implemented using Java programming. Minimization of Metabolic Adjustment (MOMA) algorithm [31] was used for glpC/b2243 gene knockout simulation.
Glycerol was chosen as the solitary carbon source under anaerobic conditions. Glycerol uptake rate was constrained to a maximum of 18.5 mmol g DW‒1 h‒1 while its corresponding oxygen uptake rate was set to be zero, as the environmental condition is anaerobic. These values were selected based on closely established experimental observations of aerobic and anaerobic growth in E. coli [11,32].
3. Results and Discussions
In this study, an E. coli genome scale model was subjected to glpC/b2243 metabolic gene knockout for increased succinate production from glycerol under anaerobic conditions. We performed in silico deletion of the glpC/b2243 gene under anaerobic conditions in order to use model-driven, targeted biological inquiry on the underlying metabolic function of the gene in relation to succinate production in E. coli from a glycerol substrate. The results indicated that the mutant model lacking the glpC gene was predicted to have increased succinate production that was 30% higher than its wild-type parent model (Table 1). In addition, the growth rate of the mutant model was about 75% of the wild type model (see Table 1). The problem of the impaired growth rate of E. coli on glycerol could be addressed by mutational inactivation of rpoC and glpk as reported recently by Palsson and colleagues [33]. Their method involves subjecting the E. coli strain to adaptive laboratory evolution on glycerol, and subsequently identifying point mutations in rpoC and glpk genes using comparative whole genome sequencing; which ultimately allows the mutant strain to gain a fast growth advantage over its wild-type counterpart on glycerol [33].
Table 1. Strain design properties of E. coli models designed from glycerol substrate using the OptFlux software platform
|
Model Strains
of E. coli used
|
Biomass (h−1)
|
% Biomass
|
Succinate (mmol g DW−1 h−1)
|
% Succinate
|
Ethanol (mmol g DW−1 h−1)
|
Acetate (mmol g DW−1 h−1)
|
Formate (mmol g DW−1 h−1)
|
|
Orth Model WT)
|
0.3959758
|
100
|
0.12733
|
100
|
6.86885
|
25.59892
|
33.99566
|
|
GlpC/b2243
|
0.29609198
|
74.77
|
0.16604
|
130.4
|
6.86208
|
25.57664
|
33.87925
|
In principle, glycerol dissimilation in E. coli central metabolic pathways uses aerobic, anaerobic and fermentative routes to produce dihydroxyacetone phosphate (DHAP). Under anaerobic condition the route to DHAP was established to be via the GlpK- GlpABC respiratory route or GldA-DhaKLM fermentative routes (Figure 1) [1]. It was previously established that for efficient utilization of glycerol for succinate production under anaerobic conditions, E. coli preferred the use of the GldA-DhaKLM fermentative route; because higher energy NADH is generated when glycerol is consumed through GldA rather than through a reduced Flavin glpD or GlpABC route (Figure 1). We took advantage of this phenomenon and performed in silico gene knockout of the glpC gene to increase the availability of the NADH required for succinate production. E. coli regulates glycerol metabolism based on environmental and genetic conditions. Glycerol-3-phosphate dehydrogenase (glpD) is only expressed under aerobic conditions (Figure 1), while, the glycerol-3-phosphate dehydrogenase operon (glpABC) is usually expressed under anaerobic conditions. The operon consists of three open reading frames, glpABC, encoding polypeptides with varying molecular weights. The glpC subunit possesses two cysteine clusters that were characterized by an iron-sulfur binding domain, while the other two polypeptides (gldAB) formed a catalytic dimer [34]. The glpC subunit was reported to have a membrane anchor binding function for the glpAB dimer [34].
The metabolic routes of glycerol dissimilation via glpK, glycerol-3-phoasphate and glpABC to form DHAP (Figure 1) occur under anaerobic condition; but the expression of glpD (Figure 1) occurs only under aerobic conditions. This route is disadvantaged for the formation of DHAP, because the simulation conducted in this study was performed under anaerobic conditions, thereby limiting the formation of DHAP, which is required for subsequent formation of phosphoenolpyruvate (PEP). We reasoned that, by knocking out the glpC gene, the metabolic route for the production of DHAP via glpK will effectively shutdown, favoring glycerol metabolism through the GldA-DhaKLM fermentative route to form DHAP under anaerobic conditions. This could be the reason why the deletion of the glpC gene in the E. coli genome scale model predicted 30% higher succinate production to than the wild-type model.
The DhakLM route has been known to utilize phosphoenolpyruvate (PEP) as a cofactor, channeling the metabolic nodes available for the fermentative glycerol metabolism to succinate (Figure 1) [1]. As a green technology, succinate production from glycerol involved a CO2 fixing step onto a 3-carbon intermediate which is subsequently converted to succinate by the TCA reductive branch [35] (Figure 1). The E. coli PEP carboxylase (ppc) is the primary native carboxylation enzyme for succinate generation, although Gonzalez and colleagues [1] argued that it is not an ideal stable route as the PEP level will be reduced drastically when the fermentative pathway involving GldA-DhaKLM is utilized [1]. In a similar study reported by the same group [1], heterologous pyruvate carboxylase (pyc) was introduced into E. coli from L. lactis in order to retain the effective activity of the GldA-DhaKLM route, thereby creating an effective node for the subsequent pyruvate conversion to succinate [1]. E. coli lacks native pyruvate carboxylase, as such, the introduction of foreign pyc gene from L. lactis was necessary to achieve higher carbon flux to succinate from pyruvate. On the contrary, the in silico metabolic glpC/b2243 gene knockout was performed and evaluated without introduction of any foreign gene; but the mutant model achieved a succinate flux increase of about 30% when compared to the wild-type parent model (Table1). On the basis of this finding, we can now hypothesize that the deletion of the glpC gene using glycerol as substrate under anaerobic condition could have generated an additional molecule of NADH and/or increased the generation of DHAP and PEP required for subsequent carboxylation to generate 30% higher succinate flux in the m
utant model. The underlying metabolic function of glpC under anaerobic condition with a glycerol substrate in relation to the generation of reducing equivalent and succinate production remains obscure.
Although, the exact mechanism involved in increasing the succinate flux by 30% is unknown, it was established that the key to increasing succinate production in E. coli was increasing the carboxylation of PEP and pyruvate to a four (4) carbon dicarboxylic acid precursor of succinate [29]. However, glycerol dissimilation in E. coli central metabolic pathways under anaerobic conditions proceeds via DHAP to PEP and pyruvate [1,5,29]. The preferred GldA-dhaKLM fermentative route may have been activated following the deletion of the anaerobic glpC gene. The 30% succinate flux increase after the glpC gene knockout could be attributed to a possible increase in the pool of PEP and pyruvate generation. The former is a precursor to succinate production via OAA by CO2 fixation with the help of E. coli native ppc (Figure 1), while the latter is required for ATP generation, necessary for cell growth and maintenance. In addition, formate is formed from pyruvate, while additional CO2 and H2 are generated through the formate hydrogen lyase (FHL) complex from formate (Figure 1) [5]. Succinate production from glycerol in E. coli involved fixing CO2 onto a 3-carbon intermediate, which is converted to succinate through the TCA cycle reductive branch [1,35]. Therefore, the CO2 generated from formate might be used to generate more succinate production via PEP to OAA by using ppc.
4. Conclusion
The generation of glycerol as a waste from the bioethanol and biodiesel industries makes it an abundant and low cost carbon source for bio-based succinate production in engineered E. coli chassis hosts. The potentials of using glycerol for succinate production in E. coli under anaerobic conditions coupled with a model-driven, targeted glpC in silico metabolic gene knockout was evaluated using the E. coli genome scale metabolic model iJO1366, with the OptFlux software platform in this work. The flux to succinate production was increased following the glpC gene deletion under anaerobic conditions with the glycerol substrate. Although, the exact metabolic mechanism of the succinate increase still remains unknown, the underlying metabolic function of this deletion could be unfolded in the future using model-guided, experimental biological inquiry. The result of this deletion is considered significant for further investigation on the succinate metabolism from glycerol by E. coli.
Conflict of Interest
All authors declare to have no conflict of interests.









 DownLoad:
DownLoad: