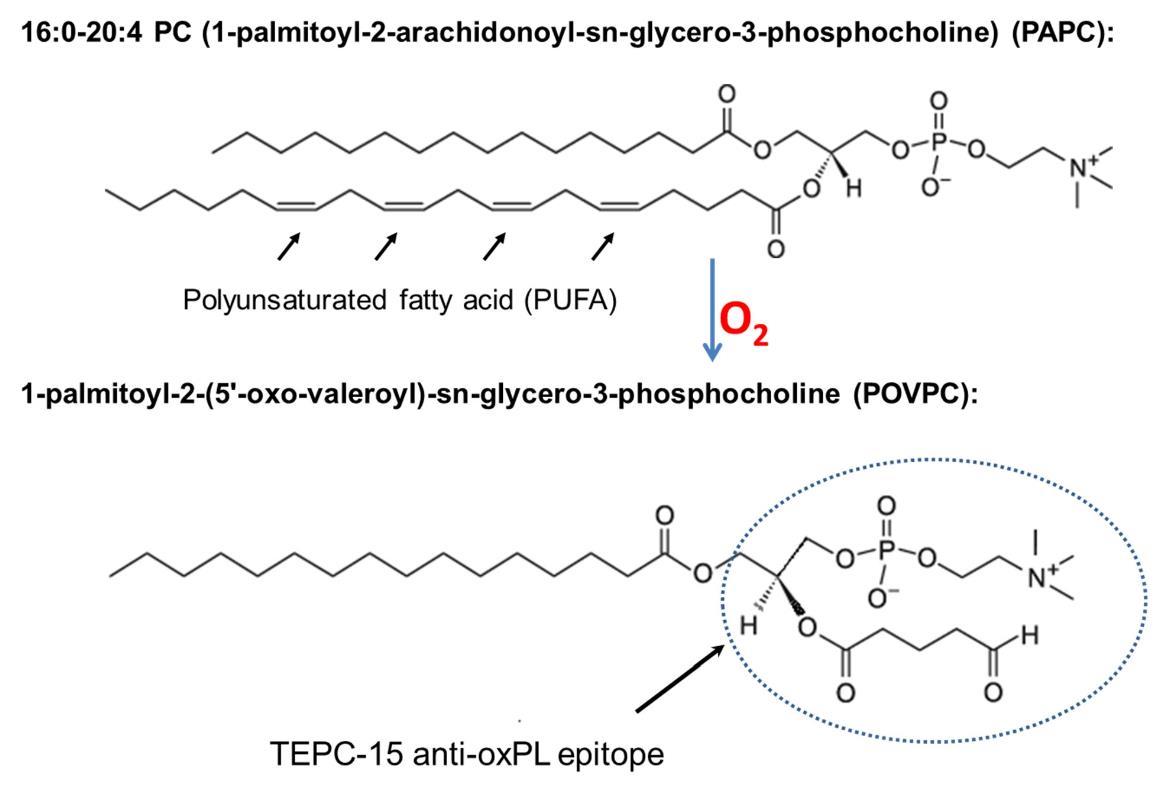
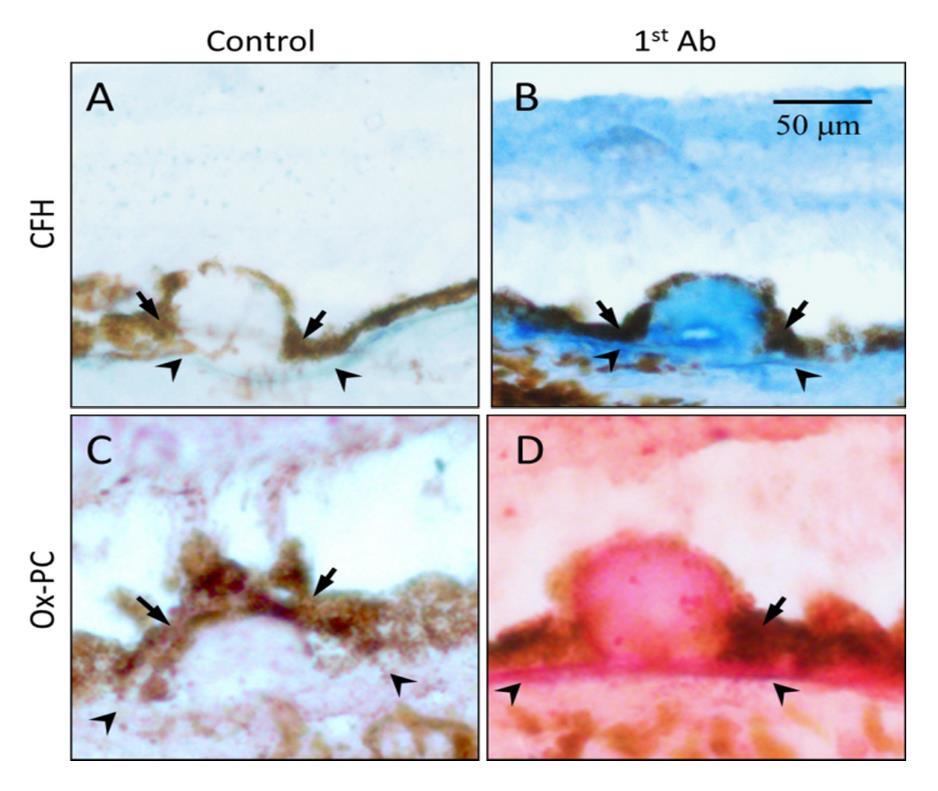
| 1.
|
Yuhai Zhao, Walter J. Lukiw,
Microbiome-Mediated Upregulation of MicroRNA-146a in Sporadic Alzheimer’s Disease,
2018,
9,
1664-2295,
10.3389/fneur.2018.00145
|
|
| 2.
|
Ting Pan, Han Shen, Songtao Yuan, Guohua Lu, Yi Zhang, Hanxue Wang, Yazhi Zhao, Xincheng Sun, Qinghuai Liu,
Combined Transplantation With Human Mesenchymal Stem Cells Improves Retinal Rescue Effect of Human Fetal RPE Cells in Retinal Degeneration Mouse Model,
2020,
61,
1552-5783,
9,
10.1167/iovs.61.8.9
|
|
| 3.
|
Leonid Minasyan, Parameswaran G. Sreekumar, David R. Hinton, Ram Kannan,
Protective Mechanisms of the Mitochondrial-Derived Peptide Humanin in Oxidative and Endoplasmic Reticulum Stress in RPE Cells,
2017,
2017,
1942-0900,
1,
10.1155/2017/1675230
|
|
| 4.
|
Ryoji Yanai, Shang Chen, Sho-Hei Uchi, Tomoaki Nanri, Kip M. Connor, Kazuhiro Kimura, Alfred S Lewin,
Attenuation of choroidal neovascularization by dietary intake of ω-3 long-chain polyunsaturated fatty acids and lutein in mice,
2018,
13,
1932-6203,
e0196037,
10.1371/journal.pone.0196037
|
|
| 5.
|
Oksana Kutsyr, Xavier Sánchez-Sáez, Natalia Martínez-Gil, Emilio de Juan, Pedro Lax, Victoria Maneu, Nicolás Cuenca,
Gradual Increase in Environmental Light Intensity Induces Oxidative Stress and Inflammation and Accelerates Retinal Neurodegeneration,
2020,
61,
1552-5783,
1,
10.1167/iovs.61.10.1
|
|
| 6.
|
Andrea Maugeri, Martina Barchitta, Maria Mazzone, Francesco Giuliano, Guido Basile, Antonella Agodi,
Resveratrol Modulates SIRT1 and DNMT Functions and Restores LINE-1 Methylation Levels in ARPE-19 Cells under Oxidative Stress and Inflammation,
2018,
19,
1422-0067,
2118,
10.3390/ijms19072118
|
|
| 7.
|
Liria Yamamoto-Rodríguez, Marco A. Zarbin, Ricardo P. Casaroli-Marano,
New frontiers and clinical implications in the pathophysiology of age-related macular degeneration,
2020,
154,
23870206,
496,
10.1016/j.medcle.2020.01.004
|
|
| 8.
|
D. V. Telegina, O. S. Kozhevnikova, N. G. Kolosova,
Changes in Retinal Glial Cells with Age and during Development of Age-Related Macular Degeneration,
2018,
83,
0006-2979,
1009,
10.1134/S000629791809002X
|
|
| 9.
|
Lindiwe M. Dlamini, Charlotte M. Tata, Marthe Carine F. Djuidje, Monisola I. Ikhile, Galina D. Nikolova, Yana D. Karamalakova, Veselina G. Gadjeva, Antoanetta M. Zheleva, Patrick B. Njobeh, Derek T. Ndinteh,
Antioxidant and prooxidant effects of Piptadeniastrum africanum as the possible rationale behind its broad scale application in African ethnomedicine,
2019,
231,
03788741,
429,
10.1016/j.jep.2018.11.039
|
|
| 10.
|
Yu Su, Yuexiong Yi, Lu Li, Changzheng Chen,
circRNA-miRNA-mRNA network in age-related macular degeneration: From construction to identification,
2021,
203,
00144835,
108427,
10.1016/j.exer.2020.108427
|
|
| 11.
|
Yeqi Zhou, Linbin Zhou, Kewen Zhou, Jingyue Zhang, Fu Shang, Xinyu Zhang,
Celastrol Protects RPE Cells from Oxidative Stress-Induced Cell Death via Activation of Nrf2 Signaling Pathway,
2019,
19,
15665240,
172,
10.2174/1566524019666190424131704
|
|
| 12.
|
Adrian Will-Orrego, Yubin Qiu, Elizabeth S. Fassbender, Siyuan Shen, Jorge Aranda, Namrata Kotagiri, Michael Maker, Sha-Mei Liao, Bruce D. Jaffee, Stephen H. Poor,
Amount of Mononuclear Phagocyte Infiltrate Does Not Predict Area of Experimental Choroidal Neovascularization (CNV),
2018,
34,
1080-7683,
489,
10.1089/jop.2017.0131
|
|
| 13.
|
Luigi Donato, Concetta Scimone, Giacomo Nicocia, Rosalia D’Angelo, Antonina Sidoti,
RETRACTED ARTICLE: Role of oxidative stress in Retinitis pigmentosa: new involved pathways by an RNA-Seq analysis,
2019,
18,
1538-4101,
84,
10.1080/15384101.2018.1558873
|
|
| 14.
|
Quiqing Zhao, Mingli Ji, Xuemei Wang,
IL-10 inhibits retinal pigment epithelium cell proliferation and migration through regulation of VEGF in rhegmatogenous retinal detachment,
2018,
1791-2997,
10.3892/mmr.2018.8787
|
|
| 15.
|
Kun Liu, Junwei Fang, Jing Jin, Shaopin Zhu, Xiaoyin Xu, Yupeng Xu, Bin Ye, Shu-Hai Lin, Xun Xu,
Serum Metabolomics Reveals Personalized Metabolic Patterns for Macular Neovascular Disease Patient Stratification,
2020,
19,
1535-3893,
699,
10.1021/acs.jproteome.9b00574
|
|
| 16.
|
Ruichan Li, Yanli Liu, Jing Xie, Xudong Huang, Li Zhang, Hua Liu, Lihua Li,
Sirt3 mediates the protective effect of hydrogen in inhibiting ROS-induced retinal senescence,
2019,
135,
08915849,
116,
10.1016/j.freeradbiomed.2019.02.005
|
|
| 17.
|
Maria Oltra, Lorena Vidal‐Gil, Rosa Maisto, Javier Sancho‐Pelluz, Jorge M. Barcia,
Oxidative stress‐induced angiogenesis is mediated by miR‐205‐5p,
2020,
24,
1582-1838,
1428,
10.1111/jcmm.14822
|
|
| 18.
|
Preeti Subramanian, S. Patricia Becerra,
2019,
Chapter 62,
978-3-030-27377-4,
377,
10.1007/978-3-030-27378-1_62
|
|
| 19.
|
Mala Upadhyay, Caroline Milliner, Brent A. Bell, Vera L. Bonilha,
Oxidative stress in the retina and retinal pigment epithelium (RPE): Role of aging, and DJ-1,
2020,
37,
22132317,
101623,
10.1016/j.redox.2020.101623
|
|
| 20.
|
Vibhuti Agrahari, Vivek Agrahari, Abhirup Mandal, Dhananjay Pal, Ashim K. Mitra,
How are we improving the delivery to back of the eye? Advances and challenges of novel therapeutic approaches,
2017,
14,
1742-5247,
1145,
10.1080/17425247.2017.1272569
|
|
| 21.
|
Maribel Vazquez,
Microfluidic and Microscale Assays to Examine Regenerative Strategies in the Neuro Retina,
2020,
11,
2072-666X,
1089,
10.3390/mi11121089
|
|
| 22.
|
Suet Ding, Suresh Kumar, Pooi Mok,
Cellular Reparative Mechanisms of Mesenchymal Stem Cells for Retinal Diseases,
2017,
18,
1422-0067,
1406,
10.3390/ijms18081406
|
|
| 23.
|
Meshal Alshamrani, Sadia Sikder, Fohona Coulibaly, Abhirup Mandal, Dhananjay Pal, Ashim K. Mitra,
Self-Assembling Topical Nanomicellar Formulation to Improve Curcumin Absorption Across Ocular Tissues,
2019,
20,
1530-9932,
10.1208/s12249-019-1404-1
|
|
| 24.
|
桥生 刘,
Advance in Risk Factors and Prevention of Dry Age-Related Macular Degeneration,
2018,
07,
2167-6542,
21,
10.12677/HJO.2018.71004
|
|
| 25.
|
The Redox Role of G6PD in Cell Growth, Cell Death, and Cancer,
2019,
8,
2073-4409,
1055,
10.3390/cells8091055
|
|
| 26.
|
Sophie C. Lee, Steven Tran, Aana Amin, Lawrence S. Morse, Ala Moshiri, Susanna S. Park, Glenn Yiu,
Retinal Vessel Density in Exudative and Nonexudative Age-Related Macular Degeneration on Optical Coherence Tomography Angiography,
2020,
212,
00029394,
7,
10.1016/j.ajo.2019.11.031
|
|
| 27.
|
Ramazan Kürşad Zor, Serpil Erşan, Erkut Küçük, Gamze Yıldırım, İsmail Sarı,
Serum malondialdehyde, monocyte chemoattractant protein-1, and vitamin C levels in wet type age-related macular degeneration patients,
2020,
12,
2515-8414,
251584142095168,
10.1177/2515841420951682
|
|
| 28.
|
Pilar Herrero-Foncubierta, Jose Paredes, Maria Giron, Rafael Salto, Juan Cuerva, Delia Miguel, Angel Orte,
A Red-Emitting, Multidimensional Sensor for the Simultaneous Cellular Imaging of Biothiols and Phosphate Ions,
2018,
18,
1424-8220,
161,
10.3390/s18010161
|
|
| 29.
|
Andrea Maugeri, Martina Barchitta, Matteo Fallico, Niccolò Castellino, Michele Reibaldi, Antonella Agodi,
Characterization of SIRT1/DNMTs Functions and LINE-1 Methylation in Patients with Age-Related Macular Degeneration,
2019,
8,
2077-0383,
159,
10.3390/jcm8020159
|
|
| 30.
|
Andrea R. Waksmunski, Robert P. Igo, Yeunjoo E. Song, Jessica N. Cooke Bailey, Renee Laux, Denise Fuzzell, Sarada Fuzzell, Larry D. Adams, Laura Caywood, Michael Prough, Dwight Stambolian, William K. Scott, Margaret A. Pericak-Vance, Jonathan L. Haines,
Rare variants and loci for age-related macular degeneration in the Ohio and Indiana Amish,
2019,
138,
0340-6717,
1171,
10.1007/s00439-019-02050-4
|
|
| 31.
|
Maarten P. Rozing, Jon A. Durhuus, Marie Krogh Nielsen, Yousif Subhi, Thomas BL. Kirkwood, Rudi GJ. Westendorp, Torben Lykke Sørensen,
Age-related macular degeneration: A two-level model hypothesis,
2020,
76,
13509462,
100825,
10.1016/j.preteyeres.2019.100825
|
|
| 32.
|
Tarek K. Abouzed, Kadry M. Sadek, Mousa M. Ayoub, Ebeed A. Saleh, Sherif M. Nasr, Yasser S. El-Sayed, Moustafa Shoukry,
Papaya extract upregulates the immune and antioxidants-related genes, and proteins expression in milk somatic cells of Friesian dairy cows,
2019,
103,
09312439,
407,
10.1111/jpn.13032
|
|
| 33.
|
Alberto Modenese, Fabriziomaria Gobba,
Macular degeneration and occupational risk factors: a systematic review,
2019,
92,
0340-0131,
1,
10.1007/s00420-018-1355-y
|
|
| 34.
|
Jasvinder A. Singh, John D. Cleveland, Der-Chong Tsai,
Gout and the risk of age-related macular degeneration in the elderly,
2018,
13,
1932-6203,
e0199562,
10.1371/journal.pone.0199562
|
|
| 35.
|
Jae Yeon Kim, Sohae Park, So Hyun Park, Dongsook Lee, Gyu Hyun Kim, Jung Eun Noh, Kea Joo Lee, Gi Jin Kim,
Overexpression of pigment epithelium-derived factor in placenta-derived mesenchymal stem cells promotes mitochondrial biogenesis in retinal cells,
2021,
101,
0023-6837,
51,
10.1038/s41374-020-0470-z
|
|
| 36.
|
Vibhuti Agrahari, Sulabh P. Patel, Nikhil Dhall, Zach Aulgur, Siddhant Thukral, Xiaoyan Yang, Ryan Conley, Ashim K. Mitra,
Nanoparticles in thermosensitive gel based composite nanosystem for ocular diseases,
2018,
8,
2190-393X,
422,
10.1007/s13346-017-0435-y
|
|
| 37.
|
Jing Li, Jiaqi He, Xiang Zhang, Jiakai Li, Peiquan Zhao, Ping Fei,
TSP1 ameliorates age-related macular degeneration by regulating the STAT3-iNOS signaling pathway,
2020,
388,
00144827,
111811,
10.1016/j.yexcr.2019.111811
|
|
| 38.
|
Reem Hasaballah Alhasani, Lincoln Biswas, Ali Mohammad Tohari, Xinzhi Zhou, James Reilly, Jian-Feng He, Xinhua Shu,
Gypenosides protect retinal pigment epithelium cells from oxidative stress,
2018,
112,
02786915,
76,
10.1016/j.fct.2017.12.037
|
|
| 39.
|
Petra Schwarzer, Despina Kokona, Andreas Ebneter, Martin S. Zinkernagel,
Effect of Inhibition of Colony-Stimulating Factor 1 Receptor on Choroidal Neovascularization in Mice,
2020,
190,
00029440,
412,
10.1016/j.ajpath.2019.10.011
|
|
| 40.
|
Xiying Mao, Ting Pan, Han Shen, Huiyu Xi, Songtao Yuan, Qinghuai Liu,
The rescue effect of mesenchymal stem cell on sodium iodate-induced retinal pigment epithelial cell death through deactivation of NF-κB-mediated NLRP3 inflammasome,
2018,
103,
07533322,
517,
10.1016/j.biopha.2018.04.038
|
|
| 41.
|
Silvia Ravera, Alfonso Esposito, Paolo Degan, Federico Caicci, Lucia Manni, Anna Liguori, Angela Bisio, Valeria Iobbi, Anna Schito, Carlo Enrico Traverso, Isabella Panfoli,
The diterpene Manool extracted from
Salvia tingitana
lowers free radical production in retinal rod outer segments by inhibiting the extramitochondrial F
1
F
o
ATP synthase
,
2021,
0263-6484,
10.1002/cbf.3618
|
|
| 42.
|
Arseny V Aybush, Alexander A Gulin, Alexander A Vasin, Alexander E Dontsov, Victor A Nadtochenko, Mikhail A Ostrovsky,
Multimodal approach to reveal the effect of light irradiation on chemical composition of lipofuscin granules of human RPE tissues,
2020,
1695,
1742-6588,
012063,
10.1088/1742-6596/1695/1/012063
|
|
| 43.
|
Sharareh Kalteh, Mostafa Saadat,
Lack of association between three common genetic variations of XPC and susceptibility to age-related macular degeneration, a preliminary study,
2020,
21,
2090-2441,
10.1186/s43042-020-00060-w
|
|
| 44.
|
Maria L. Alonso-Alonso, Girish K. Srivastava, Ricardo Usategui-Martín, Maria T. García-Gutierrez, José Carlos Pastor, Ivan Fernandez-Bueno,
Mesenchymal Stem Cell Secretome Enhancement by Nicotinamide and Vasoactive Intestinal Peptide: A New Therapeutic Approach for Retinal Degenerative Diseases,
2020,
2020,
1687-966X,
1,
10.1155/2020/9463548
|
|
| 45.
|
Daniela Calzia, Paolo Degan, Federico Caicci, Maurizio Bruschi, Lucia Manni, Luca A. Ramenghi, Giovanni Candiano, Carlo Enrico Traverso, Isabella Panfoli,
Modulation of the rod outer segment aerobic metabolism diminishes the production of radicals due to light absorption,
2018,
117,
08915849,
110,
10.1016/j.freeradbiomed.2018.01.029
|
|
| 46.
|
Maiko Maruyama-Inoue, Shin Yamane, Hisayoshi Satoh, Shimpei Sato, Kazuaki Kadonosono,
Choroidal Angioarchitecture According to Ultra-Widefield Indocyanine Green Angiography in Age-Related Macular Degeneration,
2017,
1,
2474-1264,
365,
10.1177/2474126417733161
|
|
| 47.
|
Ting Zhang, Bobak Bahrami, Ling Zhu,
2018,
Chapter 14,
978-3-319-89550-5,
273,
10.1007/978-3-319-89551-2_14
|
|
| 48.
|
Virginia Puente-Muñoz, José M. Paredes, Sandra Resa, José Damaso Vílchez, Michal Zitnan, Delia Miguel, María Dolores Girón, Juan M. Cuerva, Rafael Salto, Luis Crovetto,
New Thiol-Sensitive Dye Application for Measuring Oxidative Stress in Cell Cultures,
2019,
9,
2045-2322,
10.1038/s41598-018-38132-y
|
|
| 49.
|
Liria Yamamoto-Rodríguez, Marco A. Zarbin, Ricardo P. Casaroli-Marano,
New frontiers and clinical implications in the pathophysiology of age-related macular degeneration,
2020,
154,
00257753,
496,
10.1016/j.medcli.2020.01.023
|
|
| 50.
|
Vladimir Holan, Katerina Palacka, Barbora Hermankova,
Mesenchymal Stem Cell-Based Therapy for Retinal Degenerative Diseases: Experimental Models and Clinical Trials,
2021,
10,
2073-4409,
588,
10.3390/cells10030588
|
|
| 51.
|
Thomas J. Heesterbeek, Mansour Rouhi-Parkouhi, Stephanie J. Church, Yara T. Lechanteur, Laura Lorés-Motta, Nikolaos Kouvatsos, Simon J. Clark, Paul N. Bishop, Carel B. Hoyng, Anneke I. den Hollander, Richard D. Unwin, Anthony J. Day,
Association of plasma trace element levels with neovascular age-related macular degeneration,
2020,
201,
00144835,
108324,
10.1016/j.exer.2020.108324
|
|
| 52.
|
Reşat Duman, Ayhan Vurmaz,
Role of innate immunity and oxidative stress in steroid-induced cataracts in developing chick embryos,
2018,
37,
1556-9527,
281,
10.1080/15569527.2018.1452929
|
|
| 53.
|
R. M. Lucas, S. Yazar, A. R. Young, M. Norval, F. R. de Gruijl, Y. Takizawa, L. E. Rhodes, C. A. Sinclair, R. E. Neale,
Human health in relation to exposure to solar ultraviolet radiation under changing stratospheric ozone and climate,
2019,
18,
1474-905X,
641,
10.1039/C8PP90060D
|
|
| 54.
|
Justin Hellman, Glenn Yiu,
2018,
Chapter 2,
978-981-10-8544-4,
35,
10.1007/978-981-10-8545-1_2
|
|
| 55.
|
Ting Zhang, Mark Gillies, Ying Wang, Weiyong Shen, Bobak Bahrami, Shaoxue Zeng, Meidong Zhu, Wenjuan Yao, Fanfan Zhou, Michael Murray, Ke Wang, Ling Zhu,
Simvastatin protects photoreceptors from oxidative stress induced by all‐
trans
‐retinal, through the up‐regulation of interphotoreceptor retinoid binding protein
,
2019,
176,
0007-1188,
2063,
10.1111/bph.14650
|
|
| 56.
|
Yvette Wooff, Nilisha Fernando, Josephine H. C. Wong, Catherine Dietrich, Riemke Aggio-Bruce, Joshua A. Chu-Tan, Avril A. B. Robertson, Sarah L. Doyle, Si Ming Man, Riccardo Natoli,
Caspase-1-dependent inflammasomes mediate photoreceptor cell death in photo-oxidative damage-induced retinal degeneration,
2020,
10,
2045-2322,
10.1038/s41598-020-58849-z
|
|
| 57.
|
Svetlana Trofimova,
2020,
Chapter 1,
978-3-030-50159-4,
1,
10.1007/978-3-030-50160-0_1
|
|
| 58.
|
Silvia Ravera, Alfonso Esposito, Paolo Degan, Federico Caicci, Daniela Calzia, Eleonora Perrotta, Lucia Manni, Angela Bisio, Valeria Iobbi, Anna Schito, Carlo Enrico Traverso, Isabella Panfoli,
Sclareol modulates free radical production in the retinal rod outer segment by inhibiting the ectopic f1fo-atp synthase,
2020,
160,
08915849,
368,
10.1016/j.freeradbiomed.2020.08.014
|
|
| 59.
|
Melissa K. Jones, Bin Lu, Dawn Zhaohui Chen, Weston R. Spivia, Augustus T. Mercado, Alexander V. Ljubimov, Clive N. Svendsen, Jennifer E. Eyk, Shaomei Wang,
In Vitro and In Vivo Proteomic Comparison of Human Neural Progenitor Cell‐Induced Photoreceptor Survival,
2019,
19,
1615-9853,
1800213,
10.1002/pmic.201800213
|
|
| 60.
|
Jiaowen Xu, Yuanyuan Tu, Ying Wang, Xun Xu, Xiaodong Sun, Laiqing Xie, Qingliang Zhao, Yang Guo, Yonghui Gu, Jingxia Du, Shu Du, Manhui Zhu, E. Song,
Prodrug of epigallocatechin-3-gallate alleviates choroidal neovascularization via down-regulating HIF-1α/VEGF/VEGFR2 pathway and M1 type macrophage/microglia polarization,
2020,
121,
07533322,
109606,
10.1016/j.biopha.2019.109606
|
|
| 61.
|
Luna Krstić, María J. González-García, Yolanda Diebold,
Ocular Delivery of Polyphenols: Meeting the Unmet Needs,
2021,
26,
1420-3049,
370,
10.3390/molecules26020370
|
|
| 62.
|
Myung Hee Kim, So Yeon Kwon, So-Yeun Woo, Woo Duck Seo, Dae Yu Kim,
Antioxidative Effects of Chrysoeriol via Activation of the Nrf2 Signaling Pathway and Modulation of Mitochondrial Function,
2021,
26,
1420-3049,
313,
10.3390/molecules26020313
|
|
| 63.
|
Silvia Ravera, Federico Caicci, Paolo Degan, Davide Maggi, Lucia Manni, Alessandra Puddu, Massimo Nicolò, Carlo E. Traverso, Isabella Panfoli,
Inhibitory Action of Antidiabetic Drugs on the Free Radical Production by the Rod Outer Segment Ectopic Aerobic Metabolism,
2020,
9,
2076-3921,
1133,
10.3390/antiox9111133
|
|
| 64.
|
Soo-Young Kim, Siva P. Kambhampati, Imran A. Bhutto, D. Scott McLeod, Gerard A. Lutty, Rangaramanujam M. Kannan,
Evolution of oxidative stress, inflammation and neovascularization in the choroid and retina in a subretinal lipid induced age-related macular degeneration model,
2021,
203,
00144835,
108391,
10.1016/j.exer.2020.108391
|
|
| 65.
|
Zofia Ulańczyk, Aleksandra Grabowicz, Elżbieta Cecerska-Heryć, Daria Śleboda-Taront, Elżbieta Krytkowska, Katarzyna Mozolewska-Piotrowska, Krzysztof Safranow, Miłosz Piotr Kawa, Barbara Dołęgowska, Anna Machalińska,
Dietary and Lifestyle Factors Modulate the Activity of the Endogenous Antioxidant System in Patients with Age-Related Macular Degeneration: Correlations with Disease Severity,
2020,
9,
2076-3921,
954,
10.3390/antiox9100954
|
|
| 66.
|
Vandana Soni, Vikas Pandey, Rahul Tiwari, Saket Asati, Rakesh K. Tekade,
2019,
9780128179093,
473,
10.1016/B978-0-12-817909-3.00013-3
|
|
| 67.
|
Luis Fernando Hernández-Zimbrón, Ruben Zamora-Alvarado, Lenin Ochoa-De la Paz, Raul Velez-Montoya, Edgar Zenteno, Rosario Gulias-Cañizo, Hugo Quiroz-Mercado, Roberto Gonzalez-Salinas,
Age-Related Macular Degeneration: New Paradigms for Treatment and Management of AMD,
2018,
2018,
1942-0900,
1,
10.1155/2018/8374647
|
|
| 68.
|
Alberto Modenese, Leena Korpinen, Fabriziomaria Gobba,
Solar Radiation Exposure and Outdoor Work: An Underestimated Occupational Risk,
2018,
15,
1660-4601,
2063,
10.3390/ijerph15102063
|
|
| 69.
|
Saray Tabak, Sofia Schreiber-Avissar, Elie Beit-Yannai,
Crosstalk between MicroRNA and Oxidative Stress in Primary Open-Angle Glaucoma,
2021,
22,
1422-0067,
2421,
10.3390/ijms22052421
|
|
| 70.
|
Myung Hee Kim, Do-Hun Kim, Su Geun Yang, Dae Yu Kim,
Improved effect of a mitochondria-targeted antioxidant on hydrogen peroxide-induced oxidative stress in human retinal pigment epithelium cells,
2021,
22,
2050-6511,
10.1186/s40360-020-00471-w
|
|
| 71.
|
José Carlos Rivera, Rabah Dabouz, Baraa Noueihed, Samy Omri, Houda Tahiri, Sylvain Chemtob,
Ischemic Retinopathies: Oxidative Stress and Inflammation,
2017,
2017,
1942-0900,
1,
10.1155/2017/3940241
|
|
| 72.
|
Sven Schnichels, François Paquet-Durand, Marina Löscher, Teresa Tsai, José Hurst, Stephanie C. Joachim, Alexa Klettner,
Retina in a dish: Cell cultures, retinal explants and animal models for common diseases of the retina,
2021,
81,
13509462,
100880,
10.1016/j.preteyeres.2020.100880
|
|
| 73.
|
Xiao Liu, Liwei Zhang, Jiang-Hui Wang, Huilan Zeng, Jingling Zou, Wei Tan, Han Zhao, Yan He, Jingming Shi, Shigeo Yoshida, Yunping Li, Yedi Zhou,
Investigation of circRNA Expression Profiles and Analysis of circRNA-miRNA-mRNA Networks in an Animal (Mouse) Model of Age-Related Macular Degeneration,
2020,
45,
0271-3683,
1173,
10.1080/02713683.2020.1722179
|
|
| 74.
|
Jie Bai, Yumei Yang, Dingting Wu, Fan Yang,
SS‐31 protect retinal pigment epithelial cells from H
2
O
2
‐induced cell injury by reducing apoptosis
,
2021,
0305-1870,
10.1111/1440-1681.13484
|
|
| 75.
|
Min Jae Ju, Junghoon Kim, Sung Kyun Park, Dong Hyun Kim, Yoon-Hyeong Choi,
Long-term exposure to ambient air pollutants and age-related macular degeneration in middle-aged and older adults,
2022,
204,
00139351,
111953,
10.1016/j.envres.2021.111953
|
|
| 76.
|
NESTAN MERKVILADZE, NIKOLOZ OBOLASHVILI, TAMAR MAISURADZE,
MOLECULAR ASPECTS OF SOME RETINAL DISEASES,
2022,
15120392,
10.52340/jecm.2022.07.15
|
|
| 77.
|
Lavinia Carlini, Gabriele Tancreda, Valeria Iobbi, Federico Caicci, Silvia Bruno, Alfonso Esposito, Daniela Calzia, Stefano Benini, Angela Bisio, Lucia Manni, Anna Schito, Carlo Enrico Traverso, Silvia Ravera, Isabella Panfoli,
The Flavone Cirsiliol from Salvia x jamensis Binds the F1 Moiety of ATP Synthase, Modulating Free Radical Production,
2022,
11,
2073-4409,
3169,
10.3390/cells11193169
|
|
| 78.
|
Charles C. Wykoff, Vrinda Hershberger, David Eichenbaum, Erin Henry, Husam S. Younis, Priya Chandra, Nancy Yuan, Mark Solloway, Alex DePaoli,
Inhibition of Complement Factor 3 in Geographic Atrophy with NGM621: Phase 1 Dose-Escalation Study Results,
2022,
235,
00029394,
131,
10.1016/j.ajo.2021.08.018
|
|
| 79.
|
Minghui Wang, Won-min Song, Chen Ming, Qian Wang, Xianxiao Zhou, Peng Xu, Azra Krek, Yonejung Yoon, Lap Ho, Miranda E. Orr, Guo-Cheng Yuan, Bin Zhang,
Guidelines for bioinformatics of single-cell sequencing data analysis in Alzheimer’s disease: review, recommendation, implementation and application,
2022,
17,
1750-1326,
10.1186/s13024-022-00517-z
|
|
| 80.
|
Victoria Maneu, Pedro Lax, Antonio Miguel G. De Diego, Nicolás Cuenca, Antonio G. García,
Combined drug triads for synergic neuroprotection in retinal degeneration,
2022,
149,
07533322,
112911,
10.1016/j.biopha.2022.112911
|
|
| 81.
|
Alberto Melecchi, Rosario Amato, Dominga Lapi, Massimo Dal Monte, Dario Rusciano, Paola Bagnoli, Maurizio Cammalleri,
Increased efficacy of dietary supplement containing wax ester-rich marine oil and xanthophylls in a mouse model of dry macular degeneration,
2022,
13,
1663-9812,
10.3389/fphar.2022.1038730
|
|
| 82.
|
Alexander Wade, Rameshu Rallabandi, Steven Lucas, Catrina Oberg, Aruna Gorusupudi, Paul S. Bernstein, Jon D. Rainier,
The synthesis of the very long chain polyunsaturated fatty acid (VLC-PUFA) 32:6 n-3,
2021,
19,
1477-0520,
5563,
10.1039/D1OB00491C
|
|
| 83.
|
Fátima Milhano dos Santos, Sergio Ciordia, Joana Mesquita, João Paulo Castro de Sousa, Alberto Paradela, Cândida Teixeira Tomaz, Luís António Paulino Passarinha,
Vitreous humor proteome: unraveling the molecular mechanisms underlying proliferative and neovascular vitreoretinal diseases,
2023,
80,
1420-682X,
10.1007/s00018-022-04670-y
|
|
| 84.
|
R. M. Lucas, S. Yazar, A. R. Young, M. Norval, F. R. de Gruijl, Y. Takizawa, L. E. Rhodes, C. A. Sinclair, R. E. Neale,
Human health in relation to exposure to solar ultraviolet radiation under changing stratospheric ozone and climate,
2019,
18,
1474-905X,
641,
10.1039/c8pp90060d
|
|
| 85.
|
Justin C. Muste, Matthew W. Russell, Andrew X. Chen, Kanika Seth, Amogh I. Iyer, Carolina C. S. Valentim, Anna K. Wu, Blanche L. Kuo, Aneesha Kalur, Resya Sastry, Grant L. Hom, Thais F. Conti, Collin A. Rich, Katherine E. Talcott, Sumit Sharma, Rishi P. Singh,
Functional Imaging of Mitochondria in Age-Related Macular Degeneration Using Flavoprotein Fluorescence,
2023,
54,
2325-8160,
24,
10.3928/23258160-20221214-03
|
|
| 86.
|
Jun Wang, Mengling Li, Ziyue Geng, Saadullah Khattak, Xinying Ji, Dongdong Wu, Yalong Dang, Francisco Rios,
Role of Oxidative Stress in Retinal Disease and the Early Intervention Strategies: A Review,
2022,
2022,
1942-0994,
1,
10.1155/2022/7836828
|
|
| 87.
|
Zhongjie Fu, Raffael Liegl, Zhongxiao Wang, Yan Gong, Chi-Hsiu Liu, Ye Sun, Bertan Cakir, Samuel B. Burnim, Steven S. Meng, Chatarina Löfqvist, John Paul SanGiovanni, Ann Hellström, Lois E. H. Smith,
Adiponectin Mediates Dietary Omega-3 Long-Chain Polyunsaturated Fatty Acid Protection Against Choroidal Neovascularization in Mice,
2017,
58,
1552-5783,
3862,
10.1167/iovs.17-21796
|
|
| 88.
|
Kristin Krueger, Elke Boehme, Alexa Karina Klettner, Marietta Zille,
The potential of marine resources for retinal diseases: a systematic review of the molecular mechanisms,
2022,
62,
1040-8398,
7518,
10.1080/10408398.2021.1915242
|
|
| 89.
|
Ayat Mahmoud Domouky, Walaa M. Samy, Walaa A. Rashad,
Therapeutic effect of the mesenchymal stem cells on vigabatrin-induced retinopathy in adult male albino rat,
2022,
55,
2093-3665,
217,
10.5115/acb.22.006
|
|
| 90.
|
Zhijie Wang, Yinhua Huang, Feixue Chu, Kai Liao, Zekai Cui, Jiansu Chen, Shibo Tang,
Integrated Analysis of DNA methylation and transcriptome profile to identify key features of age-related macular degeneration,
2021,
12,
2165-5979,
7061,
10.1080/21655979.2021.1976502
|
|
| 91.
|
Marina Yakovleva, Alexander Dontsov, Natalia Trofimova, Natalia Sakina, Alexey Kononikhin, Arseny Aybush, Alexander Gulin, Tatiana Feldman, Mikhail Ostrovsky,
Lipofuscin Granule Bisretinoid Oxidation in the Human Retinal Pigment Epithelium forms Cytotoxic Carbonyls,
2021,
23,
1422-0067,
222,
10.3390/ijms23010222
|
|
| 92.
|
Kathleen Romond, Minhaj Alam, Sasha Kravets, Luis de Sisternes, Theodore Leng, Jennifer I Lim, Daniel Rubin, Joelle A Hallak,
Imaging and artificial intelligence for progression of age-related macular degeneration,
2021,
246,
1535-3702,
2159,
10.1177/15353702211031547
|
|
| 93.
|
Fukutaro Mano, Shoei Sakata, Kuo-Chung Chang, Tomiya Mano,
Effects of Zinc Acetate Hydrate Treatment on Serum Oxidative Stress Markers in Patients with Macular Drusen,
2021,
37,
1080-7683,
518,
10.1089/jop.2021.0031
|
|
| 94.
|
Shan-Shan Li, Hui-Hui Wang, Dawei Zhang,
Efficacy of different nutrients in age-related macular degeneration: A systematic review and network meta-analysis,
2022,
37,
0882-0538,
515,
10.1080/08820538.2021.2022165
|
|
| 95.
|
Alexa Klettner, Johann Roider,
Retinal Pigment Epithelium Expressed Toll-like Receptors and Their Potential Role in Age-Related Macular Degeneration,
2021,
22,
1422-0067,
8387,
10.3390/ijms22168387
|
|
| 96.
|
Julia Hildebrandt, Tom Käckenmeister, Katrin Winkelmann, Philipp Dörschmann, Johann Roider, Alexa Klettner,
Pro-inflammatory activation changes intracellular transport of bevacizumab in the retinal pigment epithelium in vitro,
2022,
260,
0721-832X,
857,
10.1007/s00417-021-05443-2
|
|
| 97.
|
Malgorzata Mrowicka, Jerzy Mrowicki, Ewa Kucharska, Barbara Smigielska, Jacek Pawel Szaflik, Jerzy Szaflik, Ireneusz Majsterek,
The Role of Oxidative Stress and the Importance of miRNAs as Potential Biomarkers in the Development of Age-Related Macular Degeneration,
2021,
9,
2227-9717,
1328,
10.3390/pr9081328
|
|
| 98.
|
Iswariyaraja Sridevi Gurubaran, Hanna Heloterä, Stephen Marry, Ali Koskela, Juha M. T. Hyttinen, Jussi J. Paterno, Arto Urtti, Mei Chen, Heping Xu, Anu Kauppinen, Kai Kaarniranta,
Oxidative Stress and Mitochondrial Damage in Dry Age-Related Macular Degeneration Like NFE2L2/PGC-1α -/- Mouse Model Evoke Complement Component C5a Independent of C3,
2021,
10,
2079-7737,
622,
10.3390/biology10070622
|
|
| 99.
|
Kah-Hui Wong, Hui-Yin Nam, Sze-Yuen Lew, Murali Naidu, Pamela David, Tengku Ain Kamalden, Siti Nurma Hanim Hadie, Lee-Wei Lim,
Discovering the Potential of Natural Antioxidants in Age-Related Macular Degeneration: A Review,
2022,
15,
1424-8247,
101,
10.3390/ph15010101
|
|
| 100.
|
Heran Getachew, Blanca Chinchilla, Rosario Fernandez-Godino,
2021,
Chapter 409,
978-1-0716-2584-2,
321,
10.1007/7651_2021_409
|
|
| 101.
|
Philipp Dörschmann, Hubeydullah Akkurt, Georg Kopplin, Maria Dalgaard Mikkelsen, Anne S. Meyer, Johann Roider, Alexa Klettner,
Establishment of specific age-related macular degeneration relevant gene expression panels using porcine retinal pigment epithelium for assessing fucoidan bioactivity,
2023,
231,
00144835,
109469,
10.1016/j.exer.2023.109469
|
|
| 102.
|
Philipp Dörschmann, Charlotte Seeba, Tabea Thalenhorst, Johann Roider, Alexa Klettner,
Anti-inflammatory properties of antiangiogenic fucoidan in retinal pigment epithelium cells,
2023,
9,
24058440,
e15202,
10.1016/j.heliyon.2023.e15202
|
|
| 103.
|
Samantha Sasseville, Samira Karami, Ange Tchatchouang, Pascale Charpentier, Princia Anney, Delphine Gobert, Stéphanie Proulx,
Biomaterials used for tissue engineering of barrier-forming cell monolayers in the eye,
2023,
11,
2296-4185,
10.3389/fbioe.2023.1269385
|
|
| 104.
|
Bryanna Lee, Natalie A. Afshari, Peter X. Shaw,
Oxidative stress and antioxidants in cataract development,
2024,
35,
1040-8738,
57,
10.1097/ICU.0000000000001009
|
|
| 105.
|
Sijie Gu, Siqi Wu, Zesong Lin, Zhuo Han, Kunlun Mo, Huaxing Huang, Mingsen Li, Gen Li, Hong Ouyang, Li Wang,
Screening and evaluation of antioxidants for retinal pigment epithelial cell protection: L-ergothioneine as a novel therapeutic candidate through NRF2 activation,
2024,
242,
00144835,
109862,
10.1016/j.exer.2024.109862
|
|
| 106.
|
Silvia Ravera, Nadia Bertola, Alessandra Puddu, Silvia Bruno, Davide Maggi, Isabella Panfoli,
Crosstalk between the Rod Outer Segments and Retinal Pigmented Epithelium in the Generation of Oxidative Stress in an In Vitro Model,
2023,
12,
2073-4409,
2173,
10.3390/cells12172173
|
|
| 107.
|
2023,
21,
13040855,
10.6002/ect.2021.0360
|
|
| 108.
|
Dong Hyun Jo, Su Hyun Lee, Minsol Jeon, Chang Sik Cho, Da-Eun Kim, Hyunkyung Kim, Jeong Hun Kim,
Activation of Lysosomal Function Ameliorates Amyloid-β-Induced Tight Junction Disruption in the Retinal Pigment Epithelium,
2023,
46,
10168478,
675,
10.14348/molcells.2023.0056
|
|
| 109.
|
Suresh Khadke, Poonam Gupte, Akanksha Mourya, Amit Yadav, Sarika Mane, Asavari Joshi, Madhavi Mahajan, Manisha Mishra, Supriya Bhalerao,
Immunomodulatory effect of a proprietary polyherbal formulation on healthy participants: A single- blind, randomized, placebo- controlled, exploratory clinical study,
2023,
14,
2229-3485,
130,
10.4103/picr.picr_100_22
|
|
| 110.
|
Sandeep M. Subrahmanian, Esma I. Yerlikaya, Siddharth Sunilkumar, Allyson L. Toro, Christopher M. McCurry, Stephanie L. Grillo, Alistair J. Barber, Jeffrey M. Sundstrom, Michael D. Dennis,
Deletion of the stress response protein REDD1 prevents sodium iodate-induced RPE damage and photoreceptor loss,
2024,
2509-2723,
10.1007/s11357-024-01362-2
|
|
| 111.
|
Małgorzata B. Różanowska,
Lipofuscin, Its Origin, Properties, and Contribution to Retinal Fluorescence as a Potential Biomarker of Oxidative Damage to the Retina,
2023,
12,
2076-3921,
2111,
10.3390/antiox12122111
|
|
| 112.
|
Philipp Dörschmann, Tabea Thalenhorst, Charlotte Seeba, Marie-Theres Tischhöfer, Sandesh Neupane, Johann Roider, Susanne Alban, Alexa Klettner,
Comparison of Fucoidans from Saccharina latissima Regarding Age-Related Macular Degeneration Relevant Pathomechanisms in Retinal Pigment Epithelium,
2023,
24,
1422-0067,
7939,
10.3390/ijms24097939
|
|
| 113.
|
Alfar Ahamed, Rendy Hosea, Shourong Wu, Vivi Kasim,
The Emerging Roles of the Metabolic Regulator G6PD in Human Cancers,
2023,
24,
1422-0067,
17238,
10.3390/ijms242417238
|
|
| 114.
|
Yao Wang, Xianning Liu, Bei Wang, Hanhan Sun, Yiqian Ren, Hongbing Zhang,
Compounding engineered mesenchymal stem cell-derived exosomes: A potential rescue strategy for retinal degeneration,
2024,
173,
07533322,
116424,
10.1016/j.biopha.2024.116424
|
|
| 115.
|
Menno E. Sluijter, Alexandre Teixeira, Kris Vissers, Luis Josino Brasil, Bert van Duijn,
The Anti-Inflammatory Action of Pulsed Radiofrequency—A Hypothesis and Potential Applications,
2023,
11,
2076-3271,
58,
10.3390/medsci11030058
|
|
| 116.
|
Jae Yong Park, Jae Suk Kim, Ha Eun Sim, Seung Hyun Lee, Hyun Min Na, Min Ji Kang, Je Hyung Hwang,
Prevalence and Risk Factors of Age-Related Macular Degeneration Features Among Pilots of the Republic of Korea Air Force,
2023,
0275-004X,
10.1097/IAE.0000000000003976
|
|
| 117.
|
Iswariyaraja Sridevi Gurubaran,
Mitochondrial damage and clearance in retinal pigment epithelial cells,
2024,
102,
1755-375X,
3,
10.1111/aos.16661
|
|
| 118.
|
Dao Nguyen, Thilini Thrimawithana, Terrence J. Piva, Danilla Grando, Tien Huynh,
Benefits of plant carotenoids against age-related macular degeneration,
2023,
106,
17564646,
105597,
10.1016/j.jff.2023.105597
|
|
| 119.
|
Anindya Samanta, Amer F. Alsoudi, Ehsan Rahimy, Jay Chhablani, Christina Y. Weng,
Imaging Modalities for Dry Macular Degeneration,
2024,
64,
0020-8167,
35,
10.1097/IIO.0000000000000512
|
|
| 120.
|
Maya Carleton, Nicholas W. Oesch,
Bridging the gap of vision restoration,
2024,
18,
1662-5102,
10.3389/fncel.2024.1502473
|
|
| 121.
|
Alessandra Puddu, Massimo Nicolò, Davide C. Maggi,
Combination of Saffron (Crocus sativus), Elderberry (Sambucus nigra L.) and Melilotus officinalis Protects ARPE-19 Cells from Oxidative Stress,
2025,
26,
1422-0067,
1496,
10.3390/ijms26041496
|
|
| 122.
|
Kun‐Lin Yeh, Yu‐Hsiang Kuan, Sheng‐Wen Wu, Chen‐Yu Chiang, Chun‐Jung Chen, Wen‐Ying Chen, Chi‐Chung Chou,
Irigenin Alleviates Blue Light‐Induced Retinal Damage by Upregulating Antioxidative Defense System via Nrf2 Pathway In Vivo and In Vitro,
2025,
1520-4081,
10.1002/tox.24501
|
|
| 123.
|
Chávez García Ricardo, Ortega Camarillo Clara, Contreras Ramos Alejandra, Díaz Rosas Guadalupe, Avalos Rodríguez Alejandro, C.C.J.R. Pérez-Rivero, Vergara Onofre Marcela,
Comparison of G6PD activity with a modified hormonal environment during the critical period of hypothalamic sexual differentiation in Sprague Dawley rats,
2025,
03043940,
138206,
10.1016/j.neulet.2025.138206
|
|









 DownLoad:
DownLoad: